KIDNEY TRANSPLANT TYPES AND THE WAIT LIST
Kidney transplantation procedures can be divided into several types based on the living status of the donor and the potential relationship between donor and recipient. The two major groups include deceased donor renal transplants and living renal transplants. Deceased donor renal transplants are further divided into standard criteria (donor <50 years old), expanded criteria living donor (donor >60 years old or 50 to 60 years old with two of the following, history of hypertension, terminal creatinine >1.5, or died of a stroke), and donation after cardiac death. Living-related transplants may related or unrelated. Deceased donor renal transplantation remains the most common transplant procedure. It is important to note the type of transplant a patient receives has an important impact on patient survival, allograft organ survival, and time spent waiting for transplantation.
There are currently 123,175 people waiting for lifesaving organ transplants in the United States. Of these, 101,170 await kidney transplants. Nearly 3,000 new patients are added to the kidney waiting list each month. (7). However, the number of available organs has been outpaced by the increasing number of patients listed resulting in longer wait times. Patients who entered the waiting list after 2002 had a median waiting time of more than 3 years (8). Approximately 12 people die each day while waiting for a transplant (4). It is no surprise then that patients who have a preemptive transplant (one done before the initiation of dialysis) or spend less than 1 year on the waiting list have shown to have an improved survival following transplantation (9–11). In particular, the faster the elderly patient is transplanted, the greater the survival benefit as they are more prone to have issues arise and die on the wait list (12). Over the last decade, there have been only marginal increases in the number of deceased donors. In 2006, there were 8,024 deceased donors. This increased to 11,163 in 2013 and to 13,124 in 2014 in part due to expanding the criteria for acceptable donors (7,13). In regard to living donors, the volume of living donation in transplantation peaked in 2006 (1). Recently, there has been a decline of about 13% per year and is more pronounced among blacks, males, younger adults, siblings, and parents (13,14). In absolute numbers, in 2005, there were 6,500 living donations compared to 5,733 in 2013 (7,8).
The United Network for Organ Sharing (UNOS), which is the agency that governs transplant policy and organ distribution, recently in December of 2014 revised the system that matches kidneys from deceased donors with patients needing transplantation. This aimed to extend the length of functioning transplants, to increase the likelihood of receiving a transplant for candidates who are difficult to match, and to make better use of available kidneys through increasing organ usage and reducing the need for repeat transplants. Now, UNOS will calculate the transplant waiting time from the date the patient begins dialysis, even if he or she started dialysis before being accepted for listing at a transplant center (15). This new system gave priority to candidates whom are sensitized and have difficulty finding a human leukocyte antigen (HLA)-compatible donor and allows patients expected to live longer to receive kidneys likely to function longer.
The former standard criteria/expanded criteria donor has been replaced by the Kidney Donor Profile Index (KDPI). The KDPI is calculated as a percentage; it describes how long a deceased donor kidney is expected to function compared to all the kidneys donated in the previous year in the United States. For instance, if a donor kidney has a KDPI score of 85%, then that kidney is expected to have a shorter functional life than 85% of kidneys harvested the year before (i.e., the lower the number, the better). Roughly, a KDPI score of 0% to 20% has a graft survival of about 11 years, 21% to 85% of 9 years, and 86% to 100% of about 6 years. Expanded criteria donor kidneys are thought to be the equivalent of a KDPI score of 85% or higher (15). The group of patients that would be recommended to sign for a kidney with a KDPI of >85% would be a high-risk patient that might not be expected to survive long without a transplant (15).
Transplant candidates are also risk stratified based on their estimated posttransplant survival score. This is calculated using recipient age, diabetes status, history of previous transplantation, and duration on dialysis. Those patients in the top 20th percentile will receive kidneys with a KDPI score of <20%. This is longevity matching so providing kidney allografts expected to survive posttransplant for a longer time with individuals expected to live longer. There is concern this will bias against older recipients (15).
 TRANSPLANT OUTCOMES
TRANSPLANT OUTCOMES
In deceased donor transplantation, the 1-year allograft survival rates have gradually improved over the last decade and have reached 92% recently. This is not dramatically different from the 96.6% 1-year graft survival for all living donor transplants (13). The truly important differences between deceased donor renal transplantation and living donor renal transplantation emerge with the examination of the long-term graft survival rates. With deceased donor renal transplantation, there is a substantial fall in graft survival over time. By 5 years, deceased donor allograft survival has decreased to 72.1%, whereas living donor transplant graft survival remains at 84% (13). The results of renal transplantation using kidneys from living unrelated donors such as spouses are similar to those results obtained with living-related donors. With both deceased donor and living donor transplantation, there is a significant effect of HLA matching on allograft survival, particularly in long-term graft survival (TABLE 41.1). Five-year allograft survival of a zero-mismatched HLA deceased donor organ is 75%, whereas a six-antigen mismatched deceased donor organ has a 5-year survival of 66% (8). This can be contrasted with a two haplotype matched or (zero antigen mismatch) living donor transplant that has a 5-year survival of 84% (8). In addition to the benefits of living donor renal transplantation on allograft survival, there is a substantial benefit on patient survival as well. Patients who received deceased donor transplants in the last decade had a 10-year survival of 62.4%. This is in contrast to a 78% 10-year patient survival for recipients of living donor transplants (13). The benefits of living donor transplantation for dialysis patients can perhaps best be appreciated by noting that the outcome for both patient and allograft survival with any category of living donor transplant is superior to even well-matched deceased donor transplantation. In addition, patients able to undergo living donor transplantation can have this procedure scheduled on an elective basis and are able to avoid a prolonged period on the transplant waiting lists.

Historically, the increasing size of the wait list and wait times led to the use of the previously mentioned expanded criteria donor kidneys. Elderly patients receiving these expanded criteria organs have been shown to have improved survival compared with patients who remain on the transplant waiting list (16). These transplants do, however, have a shortened allograft survival compared with kidneys obtained from standard criteria donors.
 COMBINED KIDNEY TRANSPLANT PROCEDURES
COMBINED KIDNEY TRANSPLANT PROCEDURES
The kidney transplantation can be combined with other organs, heart, lung, liver, pancreas, and bone marrow. An extensive review of all the combined organ transplants is beyond the scope of this chapter and is reviewed elsewhere in the literature. However, we will focus on the combined pancreas and kidney transplant.
Combined kidney and pancreas transplantation for patients with ESKD and type 1 diabetes has been widely available over the past 15 years. Dialysis patients who have previously received a deceased donor or living donor kidney transplant are also potential candidates for a solitary pancreas transplant. The primary indications for pancreas transplant are type 1 diabetic or a type 2 diabetic with a progressive secondary diabetic complications or a brittle diabetic. The major benefits of kidney and pancreas transplantation are an improved quality of life, freedom from insulin therapy, the potential of avoiding additional secondary complications, and a normalization of fasting glucose with a decrease in the incidence of hypoglycemic episodes (17–19). The results of studies of secondary complications of diabetic patients undergoing pancreas transplantation are difficult to interpret in light of the fact that most of these patients have had diabetes for up to two decades. Successful pancreas transplantation is associated with normalization of serum insulin responses and a normalization of glycosylated hemoglobin. Glucose counterregulation associated with hypoglycemia improves after pancreas transplantation. The symptom recognition of hypoglycemia is restored, and dangerous episodes of hypoglycemia can be avoided. Diabetic neuropathy has been shown to be stabilized, and in some instances, autonomic and peripheral diabetic neuropathy improves (20). At present, there has been no clear benefit in halting the progression of the advanced diabetic retinopathy following pancreas transplantation. Recurrent diabetic nephropathy in the transplant kidney is prevented by pancreas transplantation and native kidney diabetic nephropathy may improve (21). In addition, some data suggest that combined kidney and pancreas transplantation may be associated with improved patient survival compared with diabetic patients who undergo kidney transplantation alone. Although the potential for significant improvement in outcome for diabetic patients with kidney disease exists, the combined kidney–pancreas transplantation procedure has an increased morbidity compared with kidney transplantation alone. Surgical complications are increased with a significant incidence of wound problems, intra-abdominal infections, recurrent urinary tract infections, and primary technical failure of the pancreas allograft. In addition, successful kidney and pancreas transplantation requires a more aggressive antirejection immunosuppression strategy and results in the increased risk of infection associated with this procedure (22,23). The decision to undergo kidney and pancreas transplantation is perhaps best made by the patient in conjunction with the patient’s nephrologist in consultation with a transplant center experienced in pancreas transplantation.
 MEDICAL EVALUATION OF THE PATIENT WITH END-STAGE KIDNEY DISEASE
MEDICAL EVALUATION OF THE PATIENT WITH END-STAGE KIDNEY DISEASE
Organ transplantation improves the duration of life for the patient with ESKD (5). This result is in part due to the selection process of healthier individuals but is also related to other issues such as progression of atherosclerotic cardiovascular disease (5). However, in the perioperative period, the relative risk of death is greater in the transplant recipient compared with the same patient remaining on dialysis. Only at the point of 3 to 4 months posttransplantation does the relative risk of death equalize between the patients remaining on dialysis and those receiving a kidney transplant. It is for this reason that a very thorough medical evaluation of each patient must be performed pretransplant to ensure that the recipient is medically and nutritionally stable to undergo the rigors of an elective surgical procedure and be capable of tolerating not only the medications but also various treatments during the posttransplantation course (TABLE 41.2).

The leading causes of death posttransplantation are cardiovascular (24,25). Hence, management strategies for patients with incipient or existing cardiovascular disease remain most important. In addition, issues relating to lung disease, malignancy, preexisting viral infections, and risk for recurrent kidney disease also play an important role in stratifying the risk among candidates and may require specific therapy before transplantation and may influence the strategy for donor selection and even immunosuppression.
Cardiovascular Disease
Cardiovascular disease is the leading cause of death and graft loss after renal transplantation (26). Almost half of deaths with graft function that occur within 30 days of transplantation are due to cardiovascular disease, primarily myocardial infarction (27). Risk stratification before transplantation is critical to avoid perioperative mortality and minimize the risk for long-term morbidity and mortality (28,29). The presence or absence and the extent of cardiovascular disease may also play a role in the decision whether to undergo elective transplantation.
Although there is a large body of literature evaluating cardiovascular risk of nontransplant populations having elective surgery (30), there is limited study of transplant populations despite the fact that they have a substantial increased risk for cardiovascular disease (24,25).
The surgical risk of patients undergoing kidney transplantation is considered intermediate (1% to 5%) (31). All patients’ evaluation should start with detailed history, careful physical examination, electrocardiogram (ECG), and chest radiograph.
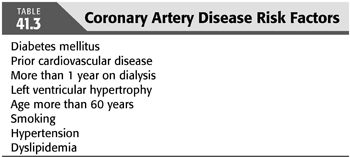
In 2012, the American Heart Association (AHA) and the American College of Cardiology (ACC) Foundation published their recommendations for evaluation and management for kidney transplant candidates. In addition to ECG, patients with three or more coronary artery risk factors (TABLE 41.3) with no active cardiac disease should be considered for noninvasive stress testing (32). The most recent European Renal Best Practice (ERBP) (33) Guidelines on the Management and Evaluation of the Kidney Donor and Recipient published in 2013 recommended performing a standard exercise tolerance test and cardiac ultrasound in asymptomatic high risk patients.
However, to this date, the optimal noninvasive test remains unclear (5).
Most of the available data on the effectiveness of noninvasive screening techniques come from studies examining either dipyridamole, thallium, or sestamibi scintigraphy (34,35), or dobutamine echocardiography (36,37). Myocardial perfusion determination with stress single photon emission computed tomographic (SPECT) imaging has proved to be helpful. If negative, 97% of patients are cardiovascular event-free with 42 months follow-up (38). Although most of the available data with these screening techniques have been in patients without ESKD, some well-defined studies have looked at patients being screened for transplantation (34–37).
Dobutamine stress echocardiography and thallium myocardial perfusion scan both have moderate sensitivity and specificity among kidney transplant candidates (32) and might even have lower accuracy for coronary artery disease (CAD) detection in dialysis patients compared to the general population (39). One systematic review suggested that dobutamine stress echocardiography is superior to myocardial perfusion scintigraphy in detecting CAD in patients who are potential kidney transplant recipients (40). In the non-ESKD population, some investigators have suggested that dobutamine echocardiography was more specific for detecting CAD than dipyridamole sestamibi scanning, whereas the sensitivities of the two tests were very similar (41). However, direct comparisons between these two screening modalities in patients with ESKD are lacking.
Echocardiography by itself is considered a reasonable test for assessing the left ventricular function in kidney transplant candidates (32). However, hemodialysis patients undergo regular hemodynamic changes that also may affect echocardiographic findings (42). Myocardial contrast echocardiography (MCE) was recently identified as a safe and uncomplicated bedside technique with good accuracy for ruling out the presence of a significant coronary artery stenosis in patients with ESKD. MCE may help along with other methods to select candidates for coronary revascularization ESKD patients (43,44).
CT scan is also not recommended for the diagnosis of CAD in dialysis patients because the correlation between coronary calcification and luminal diameter in dialysis patients is less certain than in the general population, since vascular calcification in this population is often the result of medial calcification rather than atherosclerosis (45). Stress cardiac magnetic resonance imaging (MRI) was established as valuable tool for the diagnosis and management of ischemic heart disease (46); however, experience with cardiac MRI in dialysis patients is very limited. In summary, all of these noninvasive tests are less than perfect in predicting angiographically documented coronary disease or cardiovascular events. Many still support coronary angiography as the gold standard (47).
Now, with the median waiting time for a kidney transplant is more than 3 years and can even be several years for some patients, the question of the need to repeat cardiac testing done at the time of listing or the frequency of performing such tests becomes vital. Again, to this date, there are no clear guidelines exit and most centers have their own protocols. The AHA and the ACC Foundation suggest that the usefulness of periodically screening asymptomatic kidney transplantation candidates for myocardial ischemia while on the transplant waiting list to reduce the risk of major adverse cardiac events is uncertain (32). However, an annual ECG is reasonable in all patients waiting for a transplant, and for ESKD patients with moderate aortic stenosis, an annual echocardiogram is also reasonable (32).
National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF/KDOQI) guidelines from 2005 for CAD in dialysis patients recommend for kidney transplantation candidates with normal cardiac stress testing at listing, an annual testing in those with diabetes mellitus or known CAD, testing every 2 years in those classified as “high risk,” and testing every 3 years in others (48) (TABLE 41.4). For the optimal cardiac testing method, NKF/KDOQI recommend exercise or pharmacologic stress echocardiographic or nuclear imaging tests (48).

So far, we reviewed the recommendations for initial cardiac testing and follow-up while on the waiting list. As for intervention, the AHA and the ACC Foundation recommend cardiology referral for patients with angina, patients with ejection fraction (EF) less than 50%, patients with ischemic left ventricular dilatation, and patients with established exercise-induced hypotension (TABLE 41.4). Patients who appear to have critical lesions should undergo revascularization or angioplasty, stenting, and so forth before transplantation. One study demonstrated that patients randomized to revascularization before transplantation had fewer posttransplant cardiovascular disease events compared with patients managed medically (49). These findings suggest that, in particular, diabetic patients and those with high risk for severe CAD should undergo elective revascularization before rather than after renal transplantation. However, this study was small and, therefore, cannot be generally extrapolated to the whole ESKD population group in large part because morbidity and mortality are increased in dialysis patients who undergo coronary artery bypass surgery compared with non-ESKD patients (50). The decision to perform coronary revascularization before transplantation should be considered in patients who meet the criteria for general population. In some asymptomatic transplant candidates, the risk of coronary revascularization may outweigh the risk of transplantation. These risks must be weighed by the multidisciplinary transplantation team on a case-by-case basis (32). For the choice of the optimal method for coronary revascularization in patients awaiting kidney transplant, coronary artery bypass grafting (CABG) appears to be superior to percutaneous coronary intervention (PCI) in diabetic candidates with multivessel CAD (51).
Perioperatively, patients already taking β-adrenergic blockers before renal transplantation should continue the medication perioperatively and postoperatively. For patients not on β-blockers but who have high cardiac risk (TABLE 41.3), it should be considered for initiating β-blockers preoperatively and to be continued postoperatively if no contraindication exists.
In regard to antiplatelets therapy, aspirin therapy should be continued indefinitely after renal transplantation in patients with known CAD, following the same ACC/AHA guidelines for general population with CAD.
Congestive heart failure is an important clinical consideration as part of the kidney transplant evaluation. Fifty percent of hemodialysis patients have a history of volume overload at some point during their clinical course during dialysis (52), and as many as 20% may have decreased systolic function on echocardiogram (53). However, it is important to note that many patients have left ventricular hypertrophy and diastolic dysfunction with impaired lusitropy. This interferes with ventricular filling during diastole and can ultimately lead to output failure. The rationale for treatment is entirely different depending on ventricular function. Those with systolic heart failure will require preload and afterload reduction, whereas those with diastolic dysfunction will need antihypertensive agents, especially those that slow heart rate and facilitate ventricular relaxation (53). There are no overt contraindications to transplantation in patients with left ventricular hypertrophy or ventricular dysfunction unless the EF is less than 20%. An analysis by Wali et al. (54) indicated that one important factor that reduced the likelihood of improvement in ventricular function posttransplantation was increasing time on dialysis. Consequently, an echocardiogram is an important part of the evaluation. On a positive note, renal transplantation improves ventricular function in most patients with EFs in the 20% to 40% range (54,55).
Reversible causes of myocardial dysfunction should also be identified and treated. Problems related to alcohol abuse, anemia, and hypertension need to be considered. Reversible ischemia also may impair ventricular function.
Cerebral vascular disease is also an important consideration that must be considered as part of the pretransplant evaluation. The increasing age and prevalence of hypertension and diabetes as a cause of ESKD with the associated macrovascular disease increases the likelihood of cerebral vascular disease. Patients with audible bruits or prior histories of transient ischemic attack (TIA) or stroke should have carotid artery Dopplers and consideration for carotid endarterectomy if significant disease is noted. Data from studies in the general population indicate that prophylactic surgery may be effective in selected patients, especially in those with a surgical risk of less than 3% and a greater than 60% diameter reduction on ultrasound or in those patients whose surgical risk is slightly higher, but have more substantial stenoses in the presence of contralateral internal carotid artery stenosis of greater than 75% (56). In addition, whether patients with cerebral vascular disease are symptomatic or not, aspirin prophylaxis should be considered, although there are no data on transplant patients to support its use. In the presence of chronic atrial fibrillation, anticoagulation should be considered for guideline as in the general population (57).
Patients with polycystic kidney disease as a cause of ESKD with a family history of intracranial aneurysms or with a previous episode of intracranial bleeding should undergo computed tomography (CT) scan or MRI to evaluate the presence of intracranial aneurysm (58). Those aneurysms greater than 10 mm should be considered for prophylactic surgical removal to prevent bleeding (59).
Peripheral vascular disease is common in patients with ESKD (60). Its presence may help identify patients who need more careful evaluation of potential CAD or cerebral vascular disease. Because the renal transplant is connected to the iliac vessels, it is important to know the status of vasculature. Disruption of compromised iliac flow with a kidney transplant could render more distal vascular beds to impair blood supply. Some patients may require reconstructive surgery of aortoiliac disease before or at the time of renal transplantation. Consequently, in high-risk patients, lower extremity noninvasive testing should be considered followed by angiography if there is any question concerning the adequacy of the circulation.
In summary, atherosclerotic cardiovascular disease involving the heart, brain, and peripheral vasculature is one of the most important aspects of the pretransplant evaluation. Careful evaluation of all risk factors, adequacy of treatment, and provision of optimal medical management is necessary. In addition, preoperative evaluation of all possible areas of vascular compromise should be rigorously pursued and surgically corrected if indicated.
Cancer
In the past decade, malignancy has become one of the three major causes of death after transplantation. Malignancies are responsible for 1% to 4% of all deaths in the dialysis population and 9% to 12% of deaths in the renal transplant population (61). Kidney transplant recipients have a 3- to 12-fold increased risk of developing nonlymphoid or solid organ cancers when compared to the general population (62). Consequently, careful pretransplant screening is important to rule out preexisting malignancy prior to transplant. The most important cancers to screen for pretransplant are those that occur with greater frequency in the general population including cancers of the lung, prostate, breast, and cervix, which will be discussed later in greater detail (63). What is not known is whether pretransplant screening may have a benefit in reducing the incidence of posttransplant malignancies. The recommendations for cancer screening in the posttransplant period are mostly extrapolated from the general populations and are consistent with screening guidelines in the general population, with the exception of cervical, skin, colorectal, and renal cancers (64).
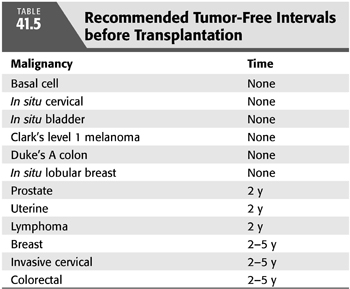
Patients with prior episodes of cancer have a waiting period before transplantation because most forms of immunosuppression will likely inhibit surveillance mechanisms that would otherwise counteract the development of a malignancy (TABLE 41.5). The Cincinnati Transplant Tumor Registry indicates that 54% of recurrences occur in patients who underwent transplantation within 2 years of treatment, whereas 33% of recurrences occurred in patients 2 to 5 years before transplantation (63). Only 13% of recurrences occurred in patients treated more than 5 years before transplantation. These statistics provide some general consideration in terms of the duration of wait between the time of treatment for cancer and transplantation. Other reports also provide some suggestions (65).
Lung cancer is the leading cause of cancer deaths (66). Screening chest x-ray film and a possible low-dose CT of the lung may be appropriate in high-risk patients with a family history who are smokers. Preferably, every patient who smokes should be made to stop before transplantation.
The overall age of male transplant recipients has risen, and it is important to consider yearly evaluation of prostate-specific antigen (PSA) and digital rectal examination before transplantation, particularly for those older than 50 years (67). This may also be even more important in the African American population in which there is a higher incidence of prostate cancer (68). If identified, it should be treated before transplantation. In the Cincinnati Transplant Tumor Registry, 40% of prostate cancer recurrences occurred within 2 years of treatment (69). However, it should be noted that the death rate associated with remaining on dialysis is often higher than death due to prostate cancer. Some investigators have developed a nomogram, based on PSA, to evaluate this risk (70), so that one can decide on an appropriate time period to delay transplantation.
The overall incidence of cervical cancers in women who received a kidney transplant is at least 2 to 3 times greater than the age- and gender-matched population. For women, a pelvic examination with cervical evaluation and manual examination of the uterus should be part of every workup and should be continued on an annual basis while on the waiting list of a transplant. In regard to human papillomavirus (HPV), studies are currently underway to determine the efficacy of HPV vaccination in women with advanced stage chronic kidney disease (CKD) and ESKD (71).
Cancer of the breast is the most common form of in situ cancer in female patients with ESKD after nonmelanoma skin cancer and cancer of the uterine cervix (72). Interestingly, renal transplant recipients appear to have a lower relative risk of breast cancer than the general population. The explanation for this may be due to improved screening or previously unidentified effects of the immunosuppression (73). Factors associated with an increased risk of recurrence posttransplantation include prior nodal involvement, bilateral disease, inflammatory carcinoma, and prior bone metastases (63). Most clinicians would recommend a waiting period of 2 years after treatment and preferably longer (5 years) for most patients given the fact that the recurrence rate may be as high as 23% and mortality associated with it is substantial (63). Biennial mammographic screening for breast cancer is standard practice in the general population. (64). Kidney Disease: Improving Global Outcomes (KDIGO) workgroup did not make a recommendation for or against mammographic screening. However, the European Best Practice Guidelines Expert Group recommended following the guidelines established for the general population (71).
Renal cell cancer is more common in the population with ESKD than in the general population, particularly in younger patients and those with ESKD from toxic, infectious, or obstructive uropathies (74). In addition, uroepithelial malignancies need to be screened for if there is any evidence of abnormalities in urinary sediment. High-risk patients may deserve an abdominal CT scan as part of their pretransplant workup.
Cutaneous malignancy is the most common form of cancer posttransplantation, particularly squamous cell carcinoma (63). Renal transplant patients have a 60- to 250-fold increased risk of developing a nonmelanoma skin cancer, which includes squamous cell carcinoma, basal cell carcinoma, Kaposi’s sarcoma, Merkel cell carcinoma, and adnexal tumors (75). There is a high recurrent rate of nonmelanoma skin cancers that occur over time after renal transplantation despite timely removal of lesions. However, this is rarely a cause of death.
Although the incidence of colorectal cancer in dialysis patients is not increased compared with the general population (76), it remains a common cancer; therefore, screening colonoscopy should be considered in all patients older than 50 years, as would be standard for the general population (72). Kidney transplant recipients appear to have a risk for colorectal cancer comparable to those in the general population 10 to 20 years older, so consideration can be given to routine screening starting at age 40 or 5 years after transplantation (77). Patients with previously treated colon cancer should wait at least 5 years before renal transplantation because the recurrence rate diminishes with time, and mortality from recurrent colon cancer after renal transplantation is very high (63).
Lymphoproliferative disorders are more common in patients with ESKD than the general population (74) and may be substantially higher in renal transplant patients if they are exposed to the Epstein-Barr virus (EBV) de novo in the posttransplantation period (78). There is a question as to whether or not prophylactic treatment with acyclovir would be capable of suppressing EBV infection and the likelihood of EBV-associated posttransplantation lymphoproliferative disease (79,80). To date, published reports supporting the potential efficacy of antiviral agents have been retrospective and have been limited by the use of either historical or no specific controls (81). In general, complete medical history and physical examination coupled with standard laboratory screening and judicious use of imaging techniques in higher risk patients should be considered as part of the standard workup. Individual determination of time to wait posttreatment of cancer before transplantation should be made on a case-by-case basis. In general, waiting at least 5 years is preferred for most previously treated cancers.
Pulmonary Disease
Anticipating respiratory complications posttransplantation in the patient with ESKD is no different than would be seen for patients without ESKD facing elective surgery. The primary focus should be elimination of smoking and evaluation of pulmonary function if there is a history of chronic obstructive pulmonary disease or other forms of lung disease that interferes with oxygenation. All patients should be screened with a careful history and physical examination and a chest x-ray film. As mentioned previously, specific focus should be addressed on the smoker because studies in the general population indicate that smokers were 5.5 times more likely to develop pulmonary complications postsurgery compared with those who did not smoke (82).
Endocrine Disease
The endocrine evaluation of a dialysis patient should primarily focus on issues surrounding diabetes, obesity, and ESKD-related bone disease. Diabetes is the leading cause of ESKD, so the proportion of patients on dialysis awaiting a transplant with diabetes is substantial. Patients with diabetes, which is most commonly type 2, have advanced risk for atherosclerotic cardiovascular disease and death (83). Consequently, all diabetic patients need careful focus on evaluation of vascular beds pretransplantation. Retinopathy, neuropathy, autonomic dysfunction, and associated complications from diabetes persist and/or progress during dialytic therapy. Despite greater likelihood of progression of complications of the diabetes posttransplantation, survival is demonstrably improved for diabetic patients with renal transplantation compared with remaining on dialysis (5).
Pancreas transplantation as discussed earlier may also be an appropriate strategy for a patient with type 1 diabetes who requires renal replacement therapy (84). With improving techniques and immunosuppression, pancreas transplantation has become an accepted therapy for patients with type 1 diabetes with patient survival rates exceeding 90% and rates of insulin independence of more than 80% at 1 year (85–87).
In the ESKD population, a higher body mass index (BMI) is associated with a reduced mortality among dialysis patients (88–90). The average cutoff for BMI for a potential transplant recipient at most transplant centers is 30 to 40. Obesity is associated with increased morbidity and mortality posttransplantation (91). Obese patients have higher rates of delayed graft function, suffer from more surgical complications and wound infections, and will frequently require prolonged hospitalization (88,92,93). Some investigators even suggest obesity is associated with increased graft failure (89), although others disagree (92). Posttransplant diabetes mellitus is more common in obese patients, and some transplant centers even suggest that increased risk of acute rejection and graft loss occurs in obese patients (91,94). In regard to weight loss prior to transplantation, transplant centers generally recommend weight loss through lifestyle and dietary changes. It is important to note that BMI should not only be looked at alone but also the overall body habitus of the patient should be taken into consideration. If a patient does not have central obesity or has a high muscle mass with a high BMI, they might not necessarily need weight loss prior to listing (95). In regard to mode of dialysis and obesity, peritoneal dialysis has not been shown to lead to greater weight gain or limit the opportunity for transplantation compared to patients on hemodialysis (96). Bariatric surgery is not routinely recommended but should be considered on a case-by-case basis particularly with recent advancements in surgical technique (95).
Pretransplant planning for the treatment of metabolic bone disease is important because preemptive strategies may prevent the development of pathologic fractures (97). However, a meta-analysis of clinical trials indicated that vitamin D and bisphosphonate therapy helps bone mineral density but did not reveal evidence of reduction in fractures (98). Patients with ESKD can suffer from high-turnover bone disease because of secondary hyperparathyroidism, low-turnover bone disease resulting from osteomalacia, or variance of both (99,100). Patients may also have dialysis-related amyloid bone disease. Renal transplantation is an effective treatment for most causes of low-turnover bone disease and for dialysis-related amyloid bone disease. However, persistence of hyperparathyroidism after successful renal transplantation is common (101). Therefore, parathyroid hormone (PTH) levels should be checked pretransplant and posttransplantation, and surgical removal may be necessary. However, since the advent of cinacalcet therapy (102), the need for parathyroidectomy has become less common.
Gastrointestinal Disease
The primary gastrointestinal issues in the patient with ESKD revolve around the presence or absence of diverticulosis or diverticulitis or other forms of colonic disease; peptic ulcer disease; gallbladder disease; or chronic liver disease, usually resulting from major hepatitis viruses or gallbladder disease.
Colon disease resulting from diverticulosis or diverticulitis is not uncommon in the patient with ESKD; in many cases, it is due to sedentary lifestyle and the medications predisposing to constipation (103). Although posttransplantation colonic perforation is a morbid and mortal consequence (104), it is unknown how to best diagnose and treat it preemptively. Patients with severe diverticulosis may require partial colectomy pretransplant. However, there is no accepted standard in terms of how to approach this problem. Patients with polycystic kidney disease have an even greater risk for colonic perforation posttransplantation, as high as 5%, which is substantially more than the 0.5% to 2% that is seen in patients with ESKD receiving renal transplantation, resulting from nonacquired polycystic kidney disease (non-APKD) (105).
Peptic ulcer disease can be a serious complication posttransplantation (106,107). The perioperative use of corticosteroids and infections such as herpes virus, cytomegalovirus (CMV), or Candida markedly increase the risk for gastritis and gastrointestinal hemorrhage. Prior history of peptic ulcer disease should prompt physicians to perform upper gastrointestinal endoscopy and fecal occult blood testing. During endoscopy, screening for Helicobacter pylori should be planned (108). With the advent of proton pump inhibitors, the risk of serious complications of gastrointestinal hemorrhage posttransplantation has markedly improved (109). Patients with a history of peptic ulcer disease have a threefold greater incidence of ulceration posttransplantation compared with those patients without a history of peptic ulcer disease (107). However, progressive screening and proton pump inhibitor use largely mitigates this risk.
All patients should have pretransplant screening for gallstones. If evident, the patient should have an elective cholecystectomy pretransplant since there is an increased risk of posttransplant complications, including cholangitis.
Liver disease is a significant cause of late morbidity and mortality among renal transplant recipients (110). In fact, death from liver failure has been reported in anywhere from 8% to 28% of renal transplant recipients (111). Chronic posttransplant liver disease is usually related to viral hepatitis, primarily hepatitis B virus (HBV) and hepatitis C virus (HCV). Serum transaminases should be routinely followed pretransplantation, and if persistently abnormal, a liver biopsy should be obtained. The primary use of the liver biopsy is to screen for cirrhosis and active hepatitis. Renal transplant recipients should be routinely screened for HBV surface antigen and HCV. Patients who are HBV surface antigen positive will also have circulating HBV antigen or serologic evidence of acute viral replication (HBV DNA) and are at increased risk of progressive liver disease posttransplantation compared to seronegative patients (112,113). All patients who are HBV surface antigen negative should receive the recombinant HBV vaccine. Although chronic dosing of lamivudine may help control viral replication in these patients, drug resistance likely limits the long-term effectiveness of this therapy (114,115).
Current guidelines recommend the use of nucleoside analogs (NA) in HBV surface antigen–positive patients preemptively before immunosuppressive therapy, regardless of baseline HBV DNA levels and for 12 months after its cessation. Lamivudine can be used only in patients with low HBV DNA (<2,000 IU/mL) and when a finite and short duration of immunosuppression is scheduled; otherwise, the candidates should be treated with a newer generation NA (116).
HCV-related liver disease is a substantial problem for hemodialysis patients because of its frequency and increased risk of death and graft failure compared to seronegative patients (117–119). Roughly 10% to 20% of hemodialysis patients are positive for HCV. Up to 50% of the cases of liver disease posttransplantation can be attributed to HCV infection (117,118). All transplant candidates should be screened for anti-HCV radioimmunoassay with confirmation testing by radioimmunoblot assay (RIBA). If positive, serum should be tested for HCV RNA to confirm current HCV infection. Serum transaminases can be followed, but a liver biopsy should be strongly considered. Patients with cirrhosis on liver biopsy should, in most instances, remain on dialysis because of an unacceptable increased risk for progressive liver failure posttransplantation (120). One exception is patients with cirrhosis but with a hepatic portal venous gradient below 10, a kidney transplant alone in this situation may be safely performed (121). Without evidence of cirrhosis, the presence of HCV antibody per se should not be a contraindication to transplantation because survival after transplantation is markedly higher than that of HCV patients who remain on chronic HD (122). Patients who are HCV RNA positive may benefit from a course of interferon-α before transplantation (123–126). Ribavirin is contraindicated in the setting of late-stage kidney disease. Thus, it would be preferable for ESKD and kidney transplant patients to have an interferon/ribavirin-free regimen. This is possible with the advent of direct-acting antivirals (DAAs). These drugs are more tolerable and avoid many of the side effects and drug interactions as compared to interferon/ribavirin. However, there presently is no recommended dosage for patients with an eGFR <30 mL/min, ideally an ESKD patient would be transplanted and would have enough renal function to dose DAA. The management of both hepatitis B and C should be done in conjunction with a hepatologist (127). This is also potentially useful to expedite a hepatitis C positive patient’s transplant as they can accept a hepatitis C positive kidney and then be treated afterward.
The goal of pretransplantation treatment is to decrease the risk of progressive liver disease and prevent the development of posttransplant HCV-associated renal disease (126). Interestingly, HCV is also associated with an increased incidence of glomerulonephritis and diabetes posttransplantation (128).
The role of immunosuppression on the progression of fibrosis in cases of HCV infection is uncertain in kidney transplant recipients. Actually, there is some evidence that cyclosporine inhibits the replication of HCV in vitro (129). It has been routine over the last decade to give HCV-positive transplant candidates HCV-positive donor kidneys (130). Most reported experiences do not suggest that there is any increased risk of progressive liver disease to the recipient in the short term (131). However, long-term observation will ultimately be necessary to demonstrate the safety of this practice. It is important to note that waiting times on the transplantation list are reduced if HCV-positive kidneys are used in HCV-infected recipients (131).
Infections
An important part of the pretransplant evaluation in the dialysis patient is to eliminate infection that may persist posttransplantation and that may become more difficult to treat or possibly become life threatening. A careful history and physical examination are important to identify possible sites of infection such as at the site of hemodialysis or peritoneal dialysis access. Peritoneal fluid should be cultured. A serologic evaluation of past viral exposures is important and may help in the design of proper posttransplantation prophylaxis treatment as well as guide decisions with regard to transplantation of a kidney from an infected donor. Transplant candidates should receive immunizations for known infections that are prevalent before transplantation, such as HBV and any childhood immunizations, that may have been missed. Seroscreening for CMV, EBV, and all the hepatitis viruses is routinely recommended. However, it is not possible to exclude possible infection with other unusual pathogens such as syphilis, strongyloidiasis, toxoplasma, or herpes. Seroscreening for HIV and tuberculin testing should also be routine.
CMV infection, until the development of effective anti-CMV drugs, was a morbid and mortal event that could occur in the transplant recipient. The incidence of CMV disease is generally less than 5% for recipients who do not have antibodies to CMV and who receive kidneys from donors who are antibody negative (132,133). However, the incidence of primary CMV disease among antibody-negative recipients of CMV-positive kidneys is high, on the order of 50% to 75% without specific and effective prophylactic regimens (132,133). This drops to 15% to 35% when using a prophylactic regimen (134). CMV disease in antibody-positive recipients receiving either positive or negative donor kidneys is approximately 25% to 40% (106,107). Therefore, this illness is frequent in the posttransplant patient and will require careful assessment of the amount of immunosuppression because this directly influences the incidence and severity of CMV disease.
A meta-analysis of controlled clinical trials demonstrated that specific antiviral agents (acyclovir or ganciclovir) were effective in preventing CMV infection in solid organ transplant recipients (135). However, it is important to note that this regimen is associated with side effects and can be expensive. Therefore, judicious and appropriate screening, pretransplantation can help identify those patients who will derive greatest benefit from the investment in prophylactic therapy. There is a concern that ganciclovir- and valganciclovir-resistant CMV strains can develop in patients receiving prophylaxis, which could undermine this therapeutic strategy (136).
Patients with ESKD are at greater risk for mycobacterial disease. This is frequently asymptomatic despite the fact that up to a third of patients with ESKD may be anergic. Purified protein derivative (PPD) testing is recommended so that isoniazid prophylaxis can be used, and performance of a chest x-ray examination can be helpful to rule out the likelihood of active infection. There is no evidence that prophylaxis with isoniazid reduces the incidence of reactivation of tuberculosis after transplantation (137). Despite this, many centers require pretransplant and/or posttransplant isoniazid prophylaxis for patients with a positive PPD skin test.
Peritoneal dialysis patients must be carefully screened for occult tunnel track or peritoneal fluid infections. In particular, Staphylococcus epidermidis may cause an occult peritonitis that can flourish once immunosuppression is started posttransplantation. Clinical studies indicate that patients on peritoneal dialysis more frequently develop infections within the first month posttransplantation compared with patients on hemodialysis. More often than not, these sites are located in the abdominal cavity, the surgical wound, or in the peritoneal fluid. Peritoneal dialysis patients with active infections should have transplantation delayed, if at all possible, if they have a history of active or recent peritonitis to ensure that proper therapy can be employed. Documentation of clearing of the infection is appropriate.
Careful evaluation of the dentition is appropriate pretransplantation in all dialysis patients. Periodontal infections and active periodontitis could worsen posttransplantation because of the use of immunosuppressive agents, particularly cyclosporine because it induces gingival hyperplasia (138). Although there are no controlled clinical trials demonstrating that treatment of periodontal disease reduces the likelihood of recurrence posttransplantation, it is appropriate to consider strategies to care for active disease processes before using medications that will stimulate gingival growth and could cover up underlying infectious processes.
Pulmonary infections posttransplantation are an important concern. Some studies suggest that renal transplant recipients develop pneumococcal infections at a rate of approximately 1% per year (139). Pneumococcal immunization is currently recommended for all chronic dialysis patients (140). It is particularly important for those who have been previously splenectomized. Annual immunization with influenza vaccine is also currently recommended for all chronic dialysis patients (140). However, there are no good studies to indicate that influenza is more severe when it occurs in an immunosuppressed transplant patient compared with a dialysis patient. Most childhood vaccinations should be employed pretransplantation, if they have never been received.
Patients who are HIV positive and have a strong desire for transplantation may be evaluated for transplantation. Data from several large prospective multicenter cohort studies have shown that solid organ transplantation in carefully selected HIV-infected individuals is safe (141). Newer approaches for immunosuppression have made transplantation feasible, and HIV is no longer an absolute contraindication for solid organ transplantation (142,143).
Genitourinary Disease
The patient with ESKD needs a careful examination of the genitourinary tract for a number of important reasons. There may be abnormalities in the drainage system that lead to the original renal dysfunction, such as bladder disease, stones, prostatic disease, or urethral strictures. These underlying abnormalities would need to be identified and corrected before the same urinary tract is used for drainage for the transplanted kidney. For this reason, a careful history and physical examination are needed. Routine recommendation for a voiding cystourethrogram (VCUG) should be considered in patients with a history of genitourinary abnormalities. Up to 25% of pretransplant VCUGs are abnormal in patients with ESKD (144). Most of the time, the abnormalities are minor and do not require surgical correction. Among younger individuals, the likelihood of congenital abnormalities is greater, whereas in older individuals, prostatic hypertrophy either from benign growth or from malignancy is a possibility. In addition, bladder cancer is 1.4 to 4.8 times more common in dialysis patients than in the general population (74). Therefore, any abnormalities on urinalysis should be carefully followed up with either a VCUG or cystoscopy. Urine cytology may also be helpful.
A neurogenic bladder is also an important problem that should be identified pretransplant. Although there are many causes, the most common are due to neurogenic issues usually related to diabetes. Patients may require intermittent catheterization or urinary diversion. In addition, patients with small bladders may need a bladder augmentation procedure or bladder stretching.
Patients with a history of urinary tract infections or stones need careful evaluation to ensure that there are no structural abnormalities that could predispose them to recurrence of these infections posttransplantation. Patients with bladder diverticula, large stones, or infected cysts, such as patients with polycystic kidney disease, may benefit from either unilateral or bilateral nephrectomy to reduce the likelihood of recurrent infection.
Other causes for a nephrectomy could include the identification of a renal cell carcinoma on ultrasound, oversized kidneys resulting from polycystic kidney disease that could impair placement of a new allograft, or possibly an inability to control blood pressure.
Recurrent Kidney Disease
It is important to identify the cause of ESKD in patients being evaluated for a kidney transplant. Almost all causes of kidney disease can recur in the kidney transplant with few exceptions such as polycystic kidney disease, Alport’s syndrome, or toxic nephropathies resulting from drug ingestion. The risk of recurrence for certain kidney diseases can be substantial and possibly lead to graft loss. In those patients who do have recurrent kidney disease, the risk of graft failure is 1.9 times higher than for patients without recurrent disease (145). In large part, the physician’s assessment of the risk of recurrence is difficult because many patients progress to ESKD without proper identification of the cause. In addition, the incidence of recurrent disease may depend on the length of follow-up, and sometimes the ability to diagnose recurrent disease from chronic allograft nephropathy may be difficult. Registry data are being collected to provide a timely understanding about their risk (146).
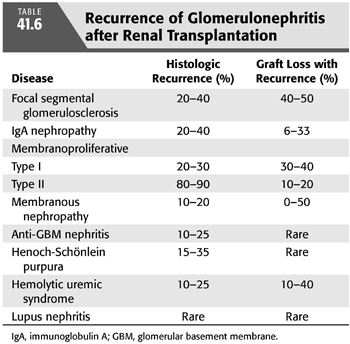
Recurrent glomerulonephritis is most commonly seen with focal segmental glomerulosclerosis (FSGS), immunoglobulin A (IgA) nephropathy, and membranoproliferative glomerulonephritis (MPGN). Other glomerulonephritides such as membranous glomerulonephritis, Wegener’s granulomatosis, and hemolytic uremic syndrome (HUS) can also recur (TABLE 41.6) (117).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


