Anthony J. Casale, MD
Posterior Urethral Valves
Description, Classification, and Embryology
Langenbeck is credited with first reporting congenital obstruction of the prostatic urethra in 1802 (Dewan et al, 1999). Despite this observation it was left for Hugh Hampton Young, over a century later, to define and name the condition as posterior urethral valves (Young et al, 1919). His remarkably accurate observations were made only with gross pathologic dissection and with the primitive cystoscopic instruments of his day. Young observed the anatomic variability of the anomaly and developed a classification that is still used almost 100 years later.
Posterior urethral valves occur in 1 in 8000 to 25,000 live births and make up 10% of urinary obstructions diagnosed in utero (Atwell, 1983; Thomas and Gordon, 1989; Casale, 1990). The diagnosis has been made on average in 1 in 1250 fetal ultrasound screenings (Gunn et al, 1995). There are reported cases of congenital urethral obstruction in females, but the classic posterior urethral valves occur only in males (Nesbit et al, 1964). The incidence of posterior urethral valves is dropping in some populations due to the effects of prenatal diagnosis and subsequent termination of affected fetuses. In one report, fetuses diagnosed with valves were electively terminated in 46% of cases (Cromie et al, 2001).
Despite several alternative proposals, Young’s classification is still most commonly used (Dewan, 1993) (Fig. 126–1). He described three types of posterior urethral valves:
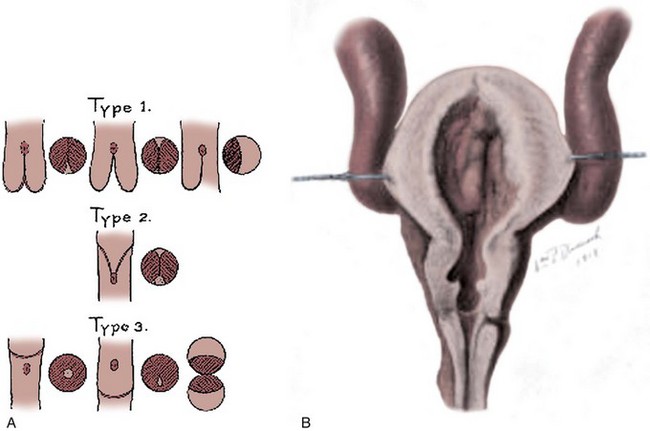
(From Young HH, Frontz WA, Baldwin JC. Congenital obstruction of the posterior urethra. J Urol 1919;3:289.)
Recently it has been proposed that all boys with valves originally have complete fusion along the anterior urethra, leaving only an open channel along the posterior urethral wall. The frequently observed cleft in the membrane may be iatrogenic, created by instrumentation or catheterization that erodes the valve tissue in the anterior midline and splits the valve in two leaflets (Robertson and Hayes, 1969; Dewan et al, 1992).
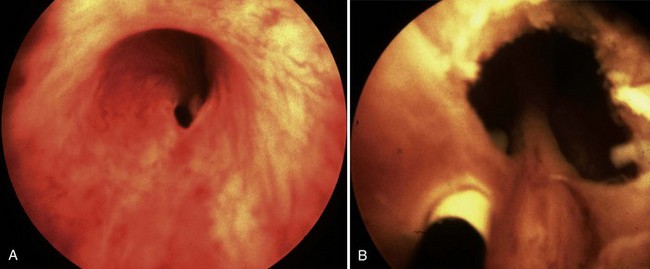
Young’s type I valves make up 95% of all posterior urethral obstructions (Fig. 126–2).
The embryology of posterior urethral valves is speculative but may be related to an abnormal insertion of the mesonephric ducts into the fetal cloaca. Evidence supporting this theory lies in the fact that type I valve patients lack plicae colliculi, mucosal folds found in the normal male urethra that are believed to represent a remnant of the normal pathway of migration of the mesonephric ducts toward the verumontanum (Stephens, 1983).
Young described type III valves as a membrane lying transversely across the urethra with a small perforation near its center (Fig. 126–3). The membrane is distal to the verumontanum and sometimes is elongated like a wind sock reaching the bulbous urethra (Field and Stephens, 1974). Type III valves make up only 5% of the total. Young described them as follows:
The embryologic origin of type III valves has been attributed to incomplete dissolution of the urogenital portion of the cloacal membrane. Type III valves present in the same manner and are managed in the same way as the more common type I, although there is some evidence that type III valves have a worse prognosis (Rosenfeld et al, 1994).
The inheritance of posterior urethral valves is poorly understood and may involve several genes and inheritance patterns (Livne et al, 1983; Weber et al, 2005). Valves have occurred in siblings, twins, and in successive generations (Farkas and Skinner, 1976; Hanlon-Lundberg et al, 1994; Morini et al, 2002; Maruotti et al, 2006). The timing of valve development is also speculative, but it would appear that they become obstructive during or after the eighth week of life by which time the prostatic urethra has developed from the urogenital sinus (Mitchell and Close, 1996). In a recent review of the literature, Baskin and colleagues concluded that the precise origins regarding the anatomy and embryology of posterior urethral valves remain undefined. Although theories of etiology abound, there are a limited number of modern methodical anatomic and histiologic studies to support them (Krishnan et al, 2006).
Pathophysiology
The effects of congenital obstruction of the urethra are reflected in the entire urinary tract above the level of obstruction (Table 126–1). Proximal urethra, prostate, bladder neck, bladder, ureters, and kidneys are all affected and suffer various forms of damage.
Table 126–1 Damage Due to Posterior Urethral Valves
| ORGAN | EFFECT | NATURAL HISTORY |
|---|---|---|
| Lung | Pulmonary hypoplasia | May be fatal in newborns; if infant survives, there are few long-term problems |
| Kidney | ||
| Glomerular injury | ||
| Obstructive uropathy | Reversible renal insufficiency | Usually improves with initial treatment but can recur with bladder dysfunction |
| Dysplasia | Irreversible renal insufficiency | Permanent level of renal damage that limits growth; leads to progressive renal failure and hypertension |
| Tubular injury | Inability to limit sodium and water loss | Progressive with age; nephrogenic diabetes insipidus |
| Bladder | Poor sensation, hypercontractility, low compliance, and eventual myogenic failure all may contribute to incontinence and poor emptying | Bladder problems are lifelong and change with age |
| Ureters | Poor contractility and inability to coapt and transport urine | Most will improve initially, but most have chronic hydronephrosis |
Lower Urinary Tract
The injury to the lower urinary tract appears to be caused by high-pressure urine storage and voiding (McConnell, 1989; Keating, 1994). Bladder histology of fetuses with posterior urethral valves shows hypertrophy and hyperplasia of the detrusor muscle along with increased connective tissue. The ratio of muscle to connective tissue is the same as in normal bladders, but there is conflicting evidence that the type of collagen within the bladder is altered (Kim et al, 1991; Ewalt et al, 1992). Some bladder findings in neonates such as wall thickness and poor compliance will improve after relief of obstruction, but the valve bladders seldom if ever achieve normal function.
High voiding pressures distend and thin the prostatic urethra. The storage capacity of the prostatic urethra sometimes exceeds that of the bladder because of the relative lack of muscle there. The verumontanum is distorted, and the ejaculatory ducts may be dilated from refluxing urine. The bladder neck is rigid and hypertrophied (Fig. 126–4). This high bladder neck was once mistaken as another cause of obstruction and was surgically incised to facilitate bladder emptying. Unfortunately, this practice often resulted in total incontinence. Today it is understood that the appearance of the bladder neck is a result of distal obstruction and not obstructive lesion itself. Bladder neck appearance and function usually improves after the obstructive valves are destroyed.
Upper Urinary Tract
Ureteral damage is usually severe. The appearance and function of the ureters are markedly abnormal. The ureteral wall is thickened and the lumen massively dilated. The ureter is at the mercy of the ureterovesical junction (UVJ). If this junction functions to prevent reflux, the ureter and kidney are somewhat protected from the complete force of the bladder contractions. If the junction allows reflux, the entire pressure of the thickened and hypercontractile bladder is transmitted directly to the upper tract with severe consequences. Virtually all valve patients have hydroureteronephrosis, and this can be the result of obstruction from the valves, the UVJ, or both. Although reflux is variable, hydroureteronephrosis is a constant in these patients. Severely dilated ureters coapt poorly and contract weakly, if at all (Gearhart et al, 1995). The propulsion of urine down the ureter is diminished in the infant, and this ureteral dysfunction may persist throughout life despite relief of distal obstruction.
The exact cause of dysplasia is a matter of continued debate. Beck and Glick demonstrated that dysplasia could be produced by early urinary obstruction in lambs (Beck, 1971; Glick et al, 1984). Maizels and colleagues (1983) also produced a form of dysplasia in chicken embryos by obstructing the fetal urinary tract. Ample evidence indicates that a form of dysplasia can be induced by early fetal urinary obstruction and resultant high intrarenal urinary pressure.
On the other hand, Stephens and Henneberry (1980) have demonstrated that dysplasia may be found in patients who do not seem to have a history of obstruction but instead have abnormal ureteral development. In patients with severe vesicoureteral reflux, the ureteral orifice is usually in an abnormal position. These patients frequently demonstrate renal dysplasia, and the degree of dysplasia is proportional to how much the position of the ureter deviates from normal. Stephens postulated that this dysplasia was the result of a faulty ureteral bud arising in an abnormal position along the mesonephric duct. Because the ureteral bud induces the development of the kidney, it follows that an abnormal bud may induce an abnormal kidney.
Dysplasia therefore occurs in association with obstruction and with reflux, seemingly unrelated conditions. Both theories are plausible and not exclusive. Whatever the cause, dysplasia is the defining factor in long-term renal function in most patients with valves. Because this type of renal damage is not reversible, the extent of dysplasia will ultimately determine the growth and functional potential of the infant’s kidneys. A number of other related factors may contribute to declining renal function in valve patients including infection, persistent obstruction, hypertension, hyperfiltration of damaged parenchyma, and perhaps high-protein diet, but none are as important as the degree of dysplasia (Brenner et al, 1982; Klahr, 1989; Farnham et al, 2005).
Tubular damage from increased urinary pressure results in failure to concentrate and acidify the urine. This occurs in up to 59% of valve patients and sometimes occurs in patients without evidence of glomerular injury (Parkhouse and Woodhouse, 1990; Dinneen et al, 1995). Concentrating defects in the form of nephrogenic diabetes insipidus lead to increased urine output independent of the patient’s state of hydration. Patients are at risk for electrolyte imbalances and dehydration due to inability to conserve sodium and water even in the face of dehydration (Gonzales, 1978). Pathologically large urine output also stresses the ureters and bladder and contributes to persistent hydroureteronephrosis and bladder dysfunction. Tubular damage worsens with age despite early relief of obstruction in many patients, and resultant high urine volumes contribute to the deterioration of renal and bladder function in late childhood.
Key Points: Pathophysiology
Vesicourethral Reflux and Dysplasia and Pop-Off Mechanisms
Hoover and Duckett identified the relationship between valves, reflux, and dysplasia (Hoover and Duckett, 1982). They recognized that valve infants with unilateral reflux had markedly different renal function when comparing refluxing and nonrefluxing kidneys. Reflux was consistently associated with dsyplastic and damaged kidneys. They postulated that the reflux allowed the high bladder pressures to focus on the refluxing kidney, sparing and protecting the nonrefluxing kidney. The vesicoureteral reflux and dysplasia (VURD) relationship is found in 13% of valve patients, and interestingly the refluxing kidney is on the left side in 92%.
The proposed mechanism of this protection is that the refluxing collecting system acts as a pressure pop-off valve. The belief was that the protected kidney would ultimately lead to a better long-term prognosis compared with boys without unilateral reflux. The long-term protective effect of VURD has not been as helpful as originally thought. Longitudinal studies in VURD patients have shown that by midchildhood both serum creatinine and glomerular filtration rate (GFR) were significantly decreased compared with normal children (Cuckow et al, 1997). In addition, scarring has been observed with dimercaptosuccinic acid scanning in half of the contralateral, nonrefluxing kidneys with possible negative long-term consequences (Narasimhan et al, 2005). Other types of pop-off pressure systems include bladder diverticuli, urinomas, and urinary ascites (Rittenberg et al, 1988). The protective effect of these clinical situations is highly variable.
Clinical Presentation
In the past boys with posterior urethral valves presented with a variety of symptoms and at various ages (Hendren, 1971). They ranged from newborns with life-threatening renal and pulmonary conditions to older children with minor voiding dysfunction. In general, the symptoms are age dependent with the more severely affected boys presenting earlier in life (Dinneen and Duffy, 1996).
Neonates
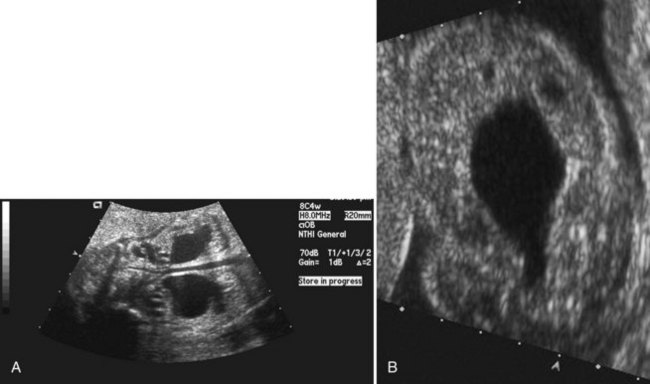
Today, most patients with posterior urethral valves are diagnosed with prenatal ultrasound. Obstruction leads to decreased fetal urine output and results in oligohydramnios. The observation of marked hydroureteronephrosis, a distended bladder, and a thickened bladder wall in utero strongly support the diagnosis of valves (Fig. 126–5). Often the urologist has been consulted long before delivery, and in some cases intervention has been undertaken in utero.
Pulmonary Hypoplasia
The most severe clinical problem facing the neonate with posterior urethral valves is pulmonary hypoplasia. The infants are often cyanotic and require respiratory support at delivery. Pulmonary hypoplasia is a direct result of oligohydramnios and accounts for most of the mortality today (Churchill et al, 1990). The exact etiology of the pulmonary hypoplasia is unclear, but contributing factors include physical restriction of fetal breathing motion (Landers and Hanson, 1990). The lack of amniotic fluid to surround and buffer the fetus from intra-abdominal pressure leads to a small chest cavity and prevents chest wall breathing motion and normal musculoskeletal development. Amniotic fluid may be important in development of the fetal pulmonary tree by providing needed intraluminal pressure, volume, and flow. In addition, the amniotic fluid may provide some cellular or molecular stimulus to the developing lung. The results of oligohydramnios are often life threatening, and some infants succumb despite heroic measures. The mortality of valves has decreased significantly over the past two decades with development of better methods to manage pulmonary hypoplasia by neonatology. It is rare to have an infant die today with the quality of pulmonary support available in the newborn intensive care unit (Dejter and Gibbons, 1989; Gibbons et al, 1993).
Renal Insufficiency
In addition to pulmonary distress, the newborn with posterior urethral valves usually presents with signs of severe systemic illness such as intrauterine growth retardation, failure to thrive, lethargy, and poor feeding. The infants may be pale and have poor muscle tone. They may demonstrate the classic signs of oligohydramnios such as Potter facies and bowed and deformed limbs with pressure dimples over knees and elbows from intrauterine pressure. Examination of the abdomen may reveal masses due to hydroureteronephrosis and a distended bladder. The infant may suffer from extensive edema and urinary ascites. The caregivers may notice a diminished force of urinary stream, but this sign is not reliable to predict valves (Smith et al, 1996). If not diagnosed in utero, the most severely affected valve patients present as newborns. Often urinary tract infection or urosepsis precipitates evaluation and diagnosis.
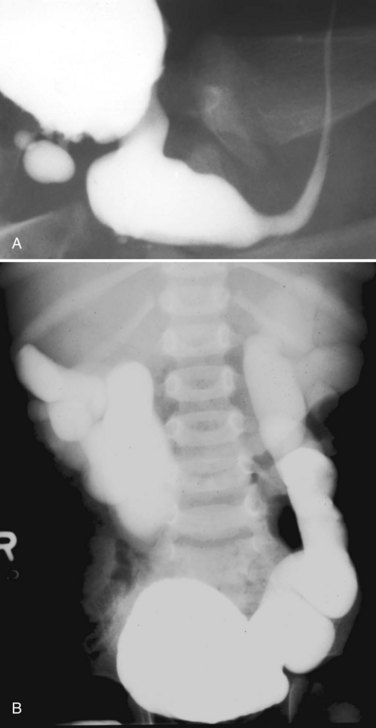
Ascites
Forty percent of neonatal ascites is caused by urinary conditions (Fig. 126–6) (Adzick et al, 1985). Urinary ascites occurs when high intraluminal pressure forces urine to extravasate from the kidney, usually across a renal fornix. Urine then enters the retroperitoneum and travels across the peritoneum as a transudate. If aspirated from the peritoneal cavity, the ascites or extravasated urine contains electrolyte and creatinine levels similar to serum. The urine within the peritoneum is subject to the large absorptive mesothelial surface that quickly normalizes these values, masking the identity of ascitic fluid as urine. The diagnosis of urinary ascites may be difficult and may require definitive upper tract drainage in the form of nephrostomy tubes in order to establish the etiology of the ascites and allow its resolution. Urinary ascites in the case of distal obstruction may serve to lower urinary pressures and offer some protection to the developing kidneys (Conner et al, 1988).
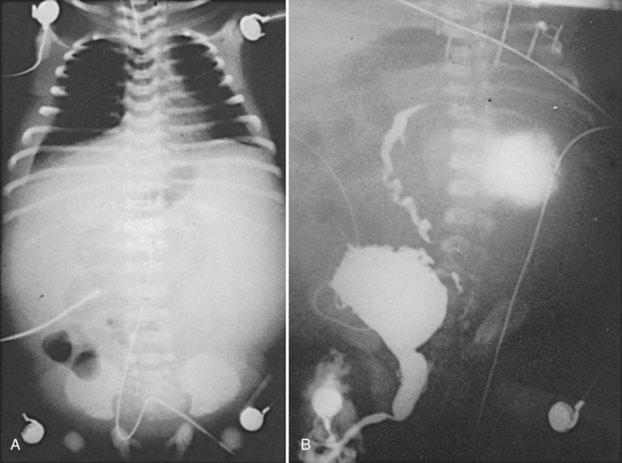
(From Gonzales ED. Posterior urethral valves and other ureteral anomalies. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campbell’s urology. 8th ed. Philadelphia: WB Saunders; 2002.)
Patel and colleagues (2003) reviewed a large group of 615 valve patients and analyzed the protective effect of ascites compared with unilateral and bilateral urinomas. The authors found that the kidney associated with a unilateral urinoma is often impaired. Bilateral urinomas are associated with good renal function, but urinary ascites alone has a poorer prognosis. The GFR of patients with urinary ascites alone was 29 mL/min per 1.73 m3 compared with 36 mL for boys with unilateral urinoma alone, 74 mL for boys with urinoma plus ascites, and 104 mL for bilateral urinomas.
Heikkila and colleagues (2008a) studied 200 patients with valves and found that the incidence of urinoma approached 15% after the introduction of ultrasound. This group included nine with perirenal urine collections, six with urinary ascites, and two with urothorax. They found initial and post-treatment serum creatinine levels, incidence of reflux, median split renal function, and risk for end-stage renal disease (ESRD) in the 17 patients with urinoma to be statistically similar to a control group of valve patients without urinoma. Their conclusion was that renal function is similar in patients with or without urinoma. Other authors have also found no association between renal function and urinoma formation in valve patients (Kleppe et al, 2006).
Older Children
The majority of boys who present later in life do so with urinary tract infection and/or voiding dysfunction. With a better understanding of functional voiding disorders, valves are now an uncommon cause of minor voiding dysfunction (Pieretti, 1993). Due to widespread prenatal ultrasound, it is now rare to see a new patient with posterior urethral valves past the neonatal period. Although boys who present later in life are generally believed to have more normal urinary tracts, this is not a uniform finding. One series reported that patients who presented at school age suffered renal insufficiency in 35% of cases (Bomalaski et al, 1999).
Initial Diagnostic Evaluation
Ultrasound
The widespread use of maternal ultrasound in the past 25 years in the United States has resulted in at least 80% of women undergoing ultrasound screening study during pregnancy. Posterior urethral valves is the third most common antenatal genitourinary diagnosis made today and accounts for 10% of all fetal uropathy (Thomas and Gordon, 1989). Although it was reported that two thirds of patients with valves presented on fetal ultrasound during the 1990s, this percentage has continued to rise (Greenfield, 1997). The quality and availability of ultrasound technology continues to improve, and with it the ability to image the fetus.
Ultrasound is sensitive in detecting fetal hydronephrosis, but the specific diagnosis of posterior urethral valves is more difficult. The differential diagnosis of bilateral hydronephrosis includes posterior urethral valves, prune-belly syndrome, bilateral ureteropelvic junction obstruction, bilateral high-grade vesicoureteral reflux, bilateral ureterovesical junction obstruction, congenital urethral atresia, and anterior urethral obstruction. Valves were correctly diagnosed in only 66% of 18 fetuses who were identified with hydronephrosis (Skolder et al, 1988). In a review of 42 patients with valves, only 45% were detected on antenatal ultrasound (Dinneen et al, 1993).
The timing of screening for fetal uropathy is an important factor in accuracy. There is compelling evidence that valves may be missed if the screening ultrasound is done before 24 weeks’ gestation (Jee et al, 1993; Hutton et al, 1994). In Dinneen’s series, 92% of the 36 patients scanned before 24 weeks’ gestation were not detected. The classic ultrasound findings in patients with valves include bilateral hydroureteronephrosis, distended bladder, dilated posterior urethra, and a thickened bladder wall. The “keyhole” sign of a dilated bladder above a dilated prostatic urethra is also helpful. Bladder wall thickness is an important diagnostic sign. The findings of an excessively thick bladder wall and a distended bladder indicate distal obstruction (Kaefer et al, 1997a).
Renal echogenicity is another parameter that may aid in the diagnosis of valves. Increased echogenicity predicted an obstructive process in a study of fetuses with bilateral hydroureteronephrosis and distended bladders (Kaefer et al, 1997b). Patients with a nonobstructive process such as megacystis-megaureter association failed to demonstrate increased echogenicity, while 87.5% of valve patients had increased echogenicity at a mean gestational age of 26 weeks. The same predictive value was found with oligohydramnios. Any male fetus with bilateral hydroureteronephrosis, decreased amniotic fluid, and a distended bladder should be considered to have posterior urethral valves until proved otherwise. The ultrasound findings of a neonate with posterior urethral valves are identical to those of a fetus.
Voiding Cystourethrography
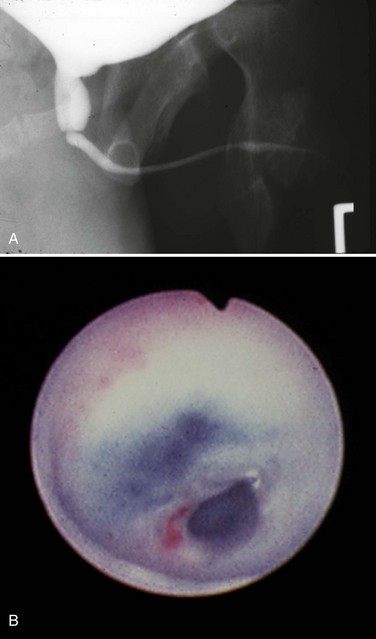
The voiding cystourethrogram (VCUG) remains the most important study in diagnosing posterior urethral valves because it defines the anatomy and gross function of the bladder, bladder neck, and urethra (Fig. 126–7). When the diagnosis of valves is in question, a VCUG should be obtained as soon as possible. On imaging the bladder and upper tracts of children with neuropathic bladder, urethral stricture, anterior urethral obstruction, and posterior urethral valves may all appear identical. Images of the urethra during voiding are necessary to make the correct diagnosis.
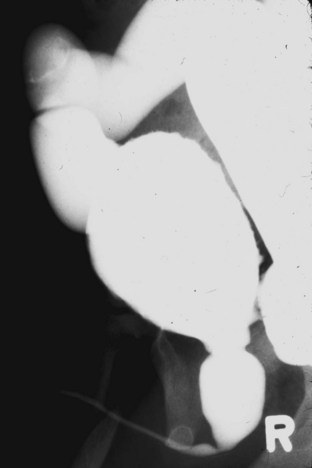
Figure 126–7 Voiding cystourethrogram reveals massive vesicoureteral reflux in an infant with posterior urethral valves.
In posterior urethral valves the bladder is thickened and trabeculated. The VCUG often demonstrates bladder diverticuli and severe vesicoureteral reflux. From the lateral projection the bladder neck is elevated. The proximal urethra is quite dilated, and the actual valve structure is often visible. Vesicoureteral reflux is present in at least 50% of valve patients at the time of diagnosis (Churchill et al, 1990). The incidence of reflux has been found to be higher in neonates than in older children. Because most valve patients are now discovered in utero, the incidence of reflux may continue to increase compared with historical studies. There is an 80% incidence of reflux on the left side in patients with unilateral reflux for no apparent reason (Hoover and Duckett, 1982).
Ultrasound imaging of the posterior urethra from the perineum has been suggested as a noninvasive way to diagnose valves (Good et al, 1996). Valve leaflets can be visualized on ultrasound while bladder wall thickness and posterior urethral diameter before and during voiding is increased in valve patients. Although this application has little to add to the newborn evaluation, it can be used to evaluate older children without catheterization.
Initial Management of Posterior Urethral Valves
Bladder Drainage
Initial management of all patients with posterior urethral valves requires the immediate establishment of urinary catheter drainage from the bladder. This should be performed even if the diagnosis has not been confirmed by VCUG. Neonates can be catheterized with a 3.5- or 5-Fr pediatric feeding tube. Foley catheters have been used with success, but there have also been reports that the balloon causes irritation and resultant bladder spasms. The bladder may be extremely irritable, and the presence of any catheter can cause spasms so severe as to obstruct the flow of urine into the bladder (Noe and Jerkins, 1983; Jordan and Hoover, 1985). Accurate catheter placement in the bladder must be documented either by irrigation of the catheter or by bladder imaging. Because it is difficult to pass the catheter over the elevated bladder neck and to avoid placing the catheter in the dilated prostatic urethra instead of the bladder, a one-shot cystogram is often advisable.
The ill newborn with posterior urethral valves is usually in the hands of the neonatal service for the management of the most threatening issues such as pulmonary hypoplasia and renal insufficiency. It is in these areas where improvement of medical management has decreased early mortality in valve babies (Nakayama et al, 1986). These infants may require maximal ventilatory support, extracorporeal membrane oxygenation, dialysis, parenteral nutrition, control of hypertension, and a host of other services provided by neonatology and pediatric nephrology (Gibbons et al, 1993).
Valve Ablation
After successful initial bladder drainage and when the patient’s medical condition has stabilized, the next step is to permanently destroy the valves. In the past, destruction of valves was done by open surgery or patients were managed with long-term suprapubic tube drainage (Gonzalez et al, 1990). Both of these early therapies can lead to unacceptable complications such as incontinence, stones, and infection (Churchill et al, 1983).
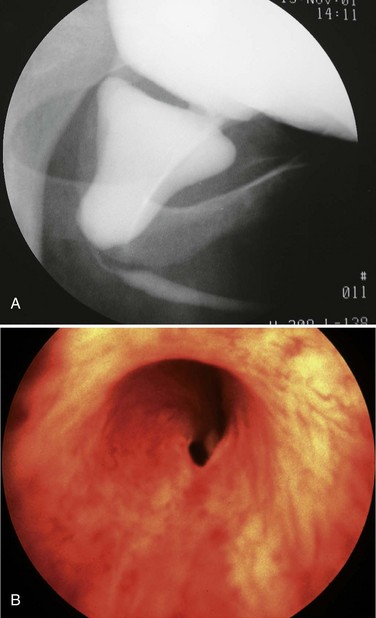
Today a number of successful surgical techniques are available to disrupt or destroy posterior urethral valves including hooks, balloon catheters, and valvulotomes (Deane et al, 1988; Kolicinski, 1988; Cromie et al, 1994; Chandna et al, 1996; Chertin et al, 2002). One of the more common methods uses the Whitaker hook. This instrument looks like a crochet hook and has been used successfully to cut valves either blindly or with fluoroscopic control (Whitaker and Sherwood, 1986). New, smaller pediatric cystoscopes with improved optics are favored today because of the ability to perform the procedure under direct vision. A bugbee electrode or a pediatric resectoscope with a hook or cold knife can be used to incise the valves (Fig. 126–8). A number of authors report using a cystoscope and laser to disrupt valves (Ehrlich and Shanberg, 1988; Biewald and Schier, 1992; Gholdoian et al, 1998). Although most surgeons incise the valves from a retrograde position viewed through the urethra, others have found that an antegrade approach through a vesicostomy or suprapubic puncture of the bladder is helpful (Zaontz and Firlit, 1985).
Originally surgeons attempted to completely resect the valves. This practice produced frequent complications from urethral stricture resulting from electrosurgical and instrument damage to the urethra. Modern appropriately sized cystoscopic equipment and limited use of electrosurgery have reduced the incidence of urethral injury to 5% (Nijman and Scholtmeyer, 1991). Today the goal is not to remove the valves but to incise them so that they are not suspended across the urethra obstructing urine flow. Well-placed incisions can disrupt their integrity and allow the valves to lie freely along the walls of the urethra when the child voids. The exact point of incision can vary; some surgeons prefer the 12 o’clock position, others prefer incisions at 4 and 8 o’clock, and others all three. Although most valves are quite thin and do not bleed at surgery, it is sometimes preferable to leave a catheter in place for 24 hours after incision. The valve remnants involute after incision, and there is often no evidence of them on later cystoscopic examination.
Documenting that the valve remnants are no longer obstructive after valve ablation is imperative. The timing of a postoperative VCUG is debatable, but one should be done to not only view the urethra but also evaluate bladder size and reflux. Decreased trabeculation, resolution of reflux, and a more uniform urethral diameter are all signs of successful relief of obstruction. One can measure the ratio of the diameter of the posterior urethra to that of the anterior urethra as an indicator of obstruction. A postablation urethral ratio of 2.5 to 3.0 is considered an acceptable postoperative result at 12 weeks (Gupta et al, 2009). Evidence indicates that early valve ablation allows the bladder to cycle, thus improving compliance and bladder stability in infancy and resulting in less bladder dysfunction in older children (Youssif et al, 2009).
Transurethral incision of the bladder neck at the time of valve incision has not been part of standard management; however, there is a report that this procedure has produced improved bladder performance with fewer inhibited contractions, lower mean maximum voiding pressures, and less need for anticholinergic medications than an age-matched control group after 4 years (Kajbafzadeh et al, 2008). Additional study of the modern use of this procedure is necessary.
Cutaneous Vesicostomy
If the infant is too small to instrument safely for valve ablation, then a cutaneous vesicostomy can be performed as a temporary measure (Fig. 126–9). Temporary vesicostomy drainage allows the urologist to incise the valves later when the patient is older and healthier. The vesicostomy has proven to be a safe and efficient treatment with long-term results in preserving renal function and somatic growth equal to primary valve ablation (Walker and Padron, 1990; Narasimhan et al, 2004). The vesicostomy provides adequate drainage of the upper tracts in more than 90% of cases (Krahn and Johnston, 1993). Vesicostomy itself is not without complication, however, and one study reported an 8.6% reoperation rate (Noe and Jerkins, 1985). Some people have questioned whether vesicostomy would cause permanent loss of bladder volume, but this has not proven to be true and vesicostomy does not significantly affect bladder capacity (Khoury et al, 1990). Some authors report that compliance may be decreased in vesicostomy bladders compared with those treated with primary ablation (Kim et al, 1996; Podesta et al, 2000). In general, primary ablation is the preferred surgical procedure to treat posterior urethral valves and vesicostomy is reserved for very small or very ill infants. Vesicostomy remains an excellent alternative treatment in these difficult situations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree