Fig. 20.1
Post-ESD scars (after curative resection of EGC)
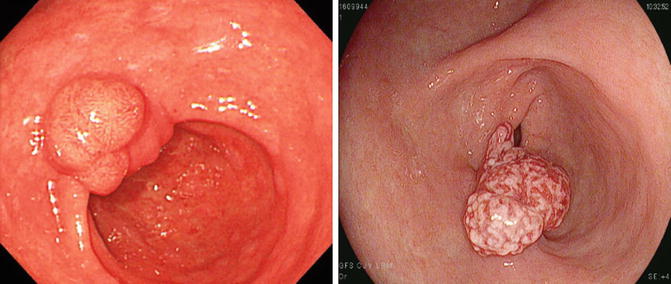
Fig. 20.2
Granulation tissue after ESD of EGC (surveillance biopsy showed no local tumor recurrence)
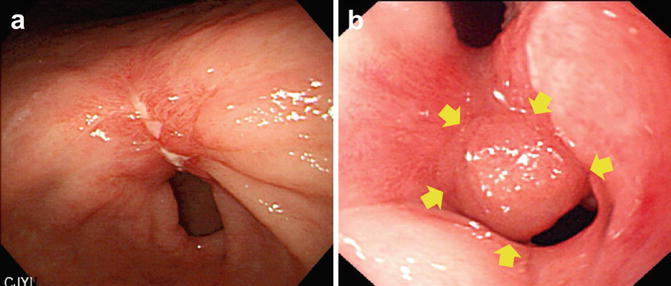
Fig. 20.3
Local recurrence at previous ESD site (after non-curative resection). (a) Irregular ulcer margin and delayed healing state (2 months after ESD). (b) Nodular lesion at ESD scar (yellow arrows)
Colorectum
The objectives of surveillance after EMR or ESD for colorectal tumors are: (1) early detection and treatment of recurrence; and (2) early detection and treatment of metachronous colorectal cancer (CRC). ESD is the most useful method for resection of large colorectal neoplasms when complete resection by EMR would not be possible. Although ESD has the potential to cure CRC, patients who undergo resection for CRC are at high risk for developing metachronous neoplasia in the remnant colorectum. Non-curative resection, tumor location, submucosal fibrosis, and piecemeal resection are associated with higher local recurrence rates. Therefore, surveillance after ESD procedure is absolutely required.
Local Recurrence
Conventional EMRs usually results in endoscopic piecemeal resections (EPMRs), particularly for large laterally spreading tumor (LST) ≥ 20 mm, with reports of local recurrence rates ranging from 7.4 to 17 % [26–28]. A case series of 67 colorectal neoplasia removed by ESD was reported [29]. The rate of en bloc resection and complete resection were 66/67 (98.5 %) and 39/67 (58.2 %), respectively. Patients who had successful en bloc resection successfully did not require additional therapy. The average duration of follow up in 48 cases was 256.1 ± 184.7 days, and no local tumor residue, metastasis, or recurrence was observed. Surgical resection and lymphadenectomy were performed for 14 lesions that indicated additional treatment, one case of local tumor residue and one case of tumor nodule in pericolic fat tissue without lymph node metastasis and local tumor residue were observed in the corresponding specimens.
Ikematsu et al. reported that for patients with early colorectal cancer, approximately 10 % of recurrences were detected within 1 year after resection, approximately 32 % within 2 years, and approximately 15 % of the recurrences after 5 years after resection [30]. Saito et al. reported 145 colorectal neoplasia cases removed by ESD. The rate of en bloc resection was 84 % (122/145), while recurrence rate was 2 %. Mean duration of recurrence detection was 6 months (2–18 months) in the EMR group and 6 months (4–6 months) in the ESD group [31]. In a systematic review, 13 series including 1,397 R0 ESD resections provided information on post-ESD follow up. Median follow up across the series was 22 months (range 6–43 months). Only one case of recurrence was reported, corresponding to a pooled risk of 0.07 % (95 % CI 0–0.2 %) [32]. ESD resulted in a significantly higher en bloc resection rate and, consequently, a significantly lower recurrence rate.
Lymph Node and Distant Metastasis
Lymph node metastasis (LNM) occurs in approximately 6–12 % of patients with submucosal invasive colorectal cancer [33–35]. According to the Paris classification and Japanese guidelines, submucosal invasive colorectal cancer lesions with well-differentiated or moderately differentiated adenocarcinoma, no evidence of vascular or lymphatic invasion, and an invasion depth of less than 1,000 μm are classified as low-risk submucosal invasive colorectal cancer, while a positive observation for any of these risk factors results in a high-risk classification of the lesion [30]. Therefore, endoscopic resection is commonly performed for low-risk submucosal invasive colorectal cancer and surgical resection with lymph node dissection is commonly performed for high-risk submucosal invasive colorectal cancer [36]. If any of the high-risk findings are observed during histopathologic examination of the endoscopic resected specimen, surgical resection with lymph node dissection is considered as an additional treatment. However, it was noted that approximately 90 % of patients with submucosal colorectal cancer with a depth of invasion of ≥1,000 μm did not have LNM. Therefore, it is important to determine whether additional treatment is indicated after sufficiently considering the depth of SM invasion in addition to whether other risk factors for lymph node metastasis are present, the physical and social background of the patient, as well as the patient’s wishes [37]. Follow-up examinations should therefore include confirmation of the presence or absence of lymph node metastasis and distant metastasis.
Metachronous Colorectal Cancer
Few reports have compared the long-term outcomes of colorectal neoplasms treated by ESD. Although both surgical and endoscopic resections have the potential to cure colorectal neoplasms, patients who undergo endoscopic resection for CRC are at a higher risk for developing metachronous neoplasia in the remnant colorectum [38, 39]. The value of intensive follow up of patients after resection of colorectal cancer remains under debate because of a lack of data. Meta-analyses of randomized controlled trials on follow up programs for patients with curatively resected colorectal cancer, however, have indicated improved overall survival and a better resection success rate for recurrent disease. Colorectal cancer surveillance colonoscopies were conducted for patients despite the long-term interval without recurrence [40, 41].
Endoscopic Follow Up
For adenomatous polyps that have been completely resected, current guidelines recommend surveillance colonoscopy 3–5 years after initial treatment. The interval is adapted in relation to the risk of developing new lesions or a late recurrence. An interval of 1–2 years is recommended for high-risk lesions which have been completely resected (high-grade intraepithelial neoplasia, villous and tubulovillous adenoma). An interval of 3 years is recommended for low-risk lesions (low-grade intraepithelial neoplasia) [42, 43]. According to data analysis between ESD and EMR, follow-up colonoscopy is recommended after 1 year for curative en bloc ESD cases considering local recurrence rates [29, 31, 32]. However, larger polyps are more likely to be incompletely resected than smaller polyps, and therefore, the US Multi-Society Task Force recommends consideration of a shorter interval for repeat colonoscopy if there is any question about completeness of resection of neoplastic tissue [44]. In clinical practice, if incomplete resection is suspected, follow-up colonoscopy can be performed 6 months after the initial ESD.
Conclusions
In patients with atrophy and intestinal metaplasia, SGNs are more frequently detected. These patients are also at higher risk for metachronous lesions. Therefore, intensive surveillance is preferred in the first year after endoscopic resection. For post-procedure surveillance, annual endoscopic examination is recommended for at least 5 years. If newly detected tumors are adequately indicated, repeat ESD can be performed. After successful ESD, the whole stomach can be preserved in many patients. In cases with non-curative resection, even closer observation, along with more surveillance biopsies, are required during endoscopic follow up.
ESD achieves a high rate of en bloc resection in patients with colorectal neoplasms. Because histopathologic diagnosis can be conducted sufficiently, the suitability of additional surgical resection can be correctly judged. Therefore, ESD is a useful method of treatment for large colorectal tumors. More outcome research and technical advances are needed, as they will play an important role in the therapeutic strategy for colorectal tumors in the future.
References
1.
2.
3.
The Japan Esophageal Society. The clinical practice guidelines for esophageal cancer (in Japanese). 3rd ed. Tokyo: Kanehara Shuppan; 2012.
4.
5.
Shimizu Y, Tukagoshi H, Fujita M, et al. Metachronous squamous cell carcinoma of the esophagus arising after endoscopic mucosal resection. Gastrointest Endosc. 2001;54:190–4.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







