Factors affecting glucose regulation
Author
Journal
Calorie restriction/Negative energy balance
Lips [65]
Isbell [66]
Lingvay [67]
Laferrere [33]
Swarbrick [68]
Clin Endocrinol, 2014
Diabetes Care, 2010
Diabetes Care, 2013
J Clin Endocrinol Metab, 2008
Diabetologia, 2008
Decrease in fat mass
Olbers [69]
Tamboli [70]
Miller [71]
Immonen [72]
Ann Surg, 2006
Obesity, 2010
Diabetes Obes Metab, 2011
J Hepatol, 2014
Decrease in lipotoxicity (adipocytokines)
Lin [73]
Malin [74]
Geloneze [75]
Diabetes, 2007
Diabetes Obes Metab, 2014
Obes Surg, 2001
Changes in hepatic glucose production
Immonen [72]
Camastra [76]
Dunn [77]
Bojsen-Moller [78]
J Hepatol, 2014
Diabetologia, 2011
Diabetes Care, 2012
Diabetes, 2014
Changes in hepatic insulin clearance
Bojsen-Moller [79]
J Clin Endocrinol Metab, 2013
Changes in insulin resistance
Camastra [76]
Dunn [77]
Bojsen-Moller [79]
Diabetologia, 2011
Diabetes Care, 2012
Diabetes, 2014
Altered glucose kinetics
Rodieux [9]
Obesity, 2008
Altered postprandial gut hormone levels (GLP-1, GIP, PYY, Ghrelin, etc.)
Rodieux [9]
Nannipieri [80]
Thaler [81]
Cummings [82]
Obesity, 2008
J Clin Endocrinol Metab, 2013
Endocrinology, 2009
N Engl J Med, 2002
Altered postprandial pancreatic hormone levels (Insulin, Glucagon, PPP)
Campos [40]
Umeda [83]
Kashyap [84]
Surg Obes Relat Dis, 2014
Obes Surg, 2011
Int J Obes, 2010
Changes in pancreatic beta-cell function
Kashyap [84]
Weiss [85]
Ferrannini [86]
Int J Obes, 2010
Diabetes, 2014
Diabetes Care, 2009
Changes in resting and meal-induced energy expenditure
Rabl [87]
Das [88]
Surgery, 2014
Am J Clin Nutr, 2003
Changes in gut microbiota
Liou [89]
Vrieze [90]
Sweeney [91]
Sci Transl Med, 2013
Gastroenterology, 2012
Best Pract Res Clin Gastroenterol, 2014
Changes in enterohepatic recirculation and bile acid composition
Sweeney [91]
Patti [92]
Pournaras [93]
Best Pract Res Clin Gastroenterol, 2014
Obesity, 2009
Endocrinology, 2012
Altered gastric emptying, nutrient intake, particle size and absorption
Carswell [94]
Obes Surg, 2014
It is important to note that earlier reports trying to elucidate the pathophysiology of the condition have suggested that, in addition to the physiologic changes described above, some patients may have developed increased pancreatic beta-cell mass or nesidioblastosis [23]. These authors hypothesized that RYGB patients with hyperinsulinemic hypoglycemia had an abnormal increase in pancreatic beta-cell mass as a result of chronic beta-cell stimulation by increased postprandial GLP-1 levels [17, 23–25]. In an often quoted publication in the New England Journal of Medicine, Service et al. [23] reported that the histologic findings of pancreatectomy specimens of patients with RYGB-related hypoglycemia as having characteristics of nesidioblastosis, including “islet cell enlargement, beta-cells budding off ductular epithelium, and islets in apposition to ducts” [5]. Based on that information, those authors and other centers then have offered subtotal or total pancreatectomy as a surgical solution for medically refractory hyperinsulinemic hypoglycemia [23–26]. However, the initial findings of the Service study have not been corroborated by other experts in the field of pancreatic beta cell replication. Meier et al. [27] demonstrated convincingly that the patients in the original publication did not have increased islet hyperplasia, greater beta-cell turn over, or greater relative beta-cell area. They showed that the original study conclusions were due to an incorrect interpretation of the pathologic findings related to an inappropriate choice of control group. In the study by Service et al., the control group was autopsy specimens from patients with pancreatic cancer, which are expected to have altered pancreatic beta-cell function and morphology [27]; and also a BMI of 33.2 to 36.3, thus substantially less obese than the index subjects before gastric bypass (BMI, 44.4 to 62.5). When Meier et al. reevaluated the same specimens from the Service study using a different control group (pancreas autopsy specimens from obese and lean subjects without pancreatic disease), they found that the pathological findings in RYGB subjects were equivalent to samples taken from obese and lean controls, thus demonstrating that there are no inherent changes in pancreatic beta-cell mass related to RYGB [27].
One aspect of pathophysiology in these patients, which has not been well studied are the changes in post-RYGB counter-regulatory mechanisms to hypoglycemia. In normal homeostasis, the body protects itself with a series of physiologic and neuroendocrine regulatory measures to maintain serum glucose levels roughly between 65 and 125 mg/dL [3]. The standard counter-regulatory mechanisms activated to respond to hypoglycemia involve multiple systems and depend on the degree of hypoglycemia. Serum glucose levels below approximately 70 mg/dL are associated with a reduction in endogenous insulin secretion and increased pancreatic glucagon production, which in turn upregulates hepatic glycogenolysis and gluconeogenesis. Serum glucose levels below 65 mg/dL promote sympathetic nervous system activation with the release of adrenaline, growth hormone, and cortisol. Prolactin, antidiuretic hormone (ADH), aldosterone, and atrial natriuretic peptide (ANP) are also released, although their contribution to glucose homeostasis is uncertain. These homeostatic mechanisms are likely also affected by the anatomic and physiologic changes, which occur after RYGB and possibly play a role in the subjects prone to develop symptomatic hyperinsulinemic hypoglycemia.
The alterations in postprandial glucose kinetics, glucose regulatory mechanisms and gastrointestinal and pancreatic hormones levels after RYGB have been extensively studied in an effort to explain diabetes remission as well as refractory RYGB-related hypoglycemia. RYGB patients have increased postprandial insulin, glucagon-like peptide-1 (GLP-1), and polypeptide YY (PYY) levels and a greater postprandial suppression of ghrelin [9]. GLP-1 is an incretin which has been identified as playing a crucial role in postprandial insulin secretion [9, 28–30] and is secreted by the L-cells of the ileum during nutrient ingestion [28]. After RYGB, patients experience postprandial increase in beta-cell secretion of insulin that is accompanied by a markedly increased secretion of GLP-1 [12, 28, 31–33]. In addition, it has been noted that there is no increase in pancreatic GLP-1 receptors in patients with RYGB-related hypoglycemia, suggesting that the pathophysiology is different from that of insulinoma [34]. Insulin response to a meal has a distinct response with a pattern of a rapid rise and peak in post-RYGB patients and this effect is exaggerated in patients who have symptomatic hypoglycemia as compared to those who are asymptomatic [29]. In addition, glucose kinetics are also altered as plasma glucose peaks earlier and higher in post-RYGB patients and are associated with lower plasma glucose nadirs [9, 35]. One recent study was performed in which patients who were administered a GLP-1 receptor blocker had significantly higher blockage of postprandial insulin secretion [29]. In fact, blockade of the GLP-1 receptor may be a potential treatment option for patients with refractory RYGB-related hypoglycemia [35].
It is clear that the etiology of symptomatic hyperinsulinemic hypoglycemia is not only due to GLP-1-stimulated insulin secretion [29]. One study found that GLP-1 was normalized after reversal of RYGB, however, hyperinsulinemic hypoglycemia persisted [32]. Other incretins, such as GIP may also play a role in post-RYGB hypoglycemia, as it has been shown to be similarly increased postprandially [31–33, 36]. Additionally, insulin sensitivity is also improved after surgery, although this result is not immediately present and requires significant weight loss to manifest [12, 37]. Nevertheless, most patients with hyperinsulinemic hypoglycemia syndrome present with symptoms once significant weight loss has occurred and thus insulin resistance is decreased.
Current evidence suggests that changes in postprandial glucagon levels do not play a role in postprandial hypoglycemia. While some have hypothesized that a lack of glucagon response to profound hypoglycemia could be attributed to the known glucagonostatic effect of the elevated GLP-1 levels [31] and that the disruption of this physiologic feedback mechanism could contribute to hypoglycemia [38], others studying glucagon levels in RYGB patients found a paradoxical increase in glucagon during OGTT after RYGB [33]. This finding has been also corroborated by our group in which five patients with well-documented hyperinsulinemic hypoglycemia syndrome had no impairment in postprandial glucagon levels and no inherent inappropriate glucagon to insulin secretion [39].
These important recent findings support the hypothesis that the pathophysiology of RYGB-related hyperinsulinemic hypoglycemia is associated with the reversible anatomic and physiologic alterations produced by RYGB and not with inherent changes in pancreatic beta-cell mass or function. This hypothesis has been tested by the documentation of normalization of glucose kinetics, abolition of neuroglycopenic episodes and normalization of postprandial levels of gastrointestinal and pancreatic hormones when a meal test is done through the excluded portion of the stomach [40, 41]. Hyperinsulinism and hypoglycemic symptoms have been shown to persist if the meal test is performed orally via the RYGB anatomy. These case series have also documented resolution of postprandial, symptomatic hyperinsulinemic hypoglycemia after RYGB in most patients after reversal to either normal anatomy or a modified sleeve gastrectomy. While RYGB reversal, as detailed below, may provide an effective surgical approach to treat this condition, much research is still underway to precisely delineate the differences between patients who are prone to developing the syndrome and those who are not. Identifying the precise mechanisms may lead to a less invasive treatment than surgical RYGB reversal.
21.3 Diagnosis
There are no clear criteria for the diagnosis of RYGB-related hyperinsulinemic hypoglycemia as the recognition of this syndrome has been evolving over the past 10 years. Here, we detail a reasonable approach to identify these patients and a diagnostic algorithm to clarify the diagnosis and rule out other causes of hypoglycemia.
The first step is recognition of the symptoms in a patient who has had RYGB. Typically, the episodes of hypoglycemia are a late complication, occurring 1–4 years after the initial surgery. As discussed later in this chapter, the symptoms can overlap with those of “dumping syndrome.” A careful history should be performed, including what symptoms are occurring, whether they coincide with a low blood sugar, what time of day they occur, whether they occur when fasting or postprandial, what foods trigger symptoms, and whether symptoms resolve with food intake. Typically, patients with RYGB-related hypoglycemia will describe symptoms beginning 1–2 h after a meal with minimal fasting symptoms. High carbohydrate intake is often a trigger for symptoms. Symptoms should improve within 15 min of food intake, but then may recur again an hour later. After many recurrent episodes of hypoglycemia, the symptoms may lessen as the patient develops hypoglycemia unawareness.
In order to diagnose hypoglycemia of any cause, it is important to identify Whipple’s triad. Whipple’s triad includes (1) the presence of classic hypoglycemia symptoms, (2) a low plasma glucose (not capillary glucometer reading) at the time of symptoms, and (3) resolution of symptoms with food intake. Therefore, laboratory testing must begin with documentation of low plasma glucose during a symptomatic episode, typically less than 55 mg/dL. Although patients may be provided with a glucometer to test capillary glucose during symptomatic episodes, these readings should not be considered diagnostic. In the case of RYGB-related hypoglycemia, symptoms are typically postprandial, so it makes sense to provoke symptoms in order to document hypoglycemia in a controlled setting. Although mixed meal tests are sometimes used, we typically perform an oral glucose tolerance test (OGTT) to assist in making the diagnosis (Fig. 21.1). The OGTT provides a higher carbohydrate load and is more likely to induce hypoglycemia and related symptoms. This provocative test can be performed on an outpatient basis and consists of administration of 50–100 g of oral glucose after a 12 h fast. The serum glucose, insulin, and C-peptide are measured at the start of the test and every 30 min thereafter for 2–3 h after administration. A typical pattern in RYGB-related hypoglycemia is a rapid rise in glucose, insulin, and C-peptide in the first 30 min, followed by a rapid decline. Although the insulin and C-peptide levels often remain elevated or inappropriately normal at the time of the hypoglycemia, they should demonstrate a rapid decrease. Persistent hyperinsulinism is not consistent with reactive hypoglycemia. Fasting hyperinsulinemia with fasting hypoglycemia would also not be expected, and would prompt consideration of alternate causes of hypoglycemia, such as insulinoma. Importantly, fasting hyperinsulinemia with normal or elevated fasting glucose is indicative of insulin resistance or diabetes, not insulinoma. A failure to see a coincident elevation in C-peptide levels would raise concern that the hypoglycemia was induced by exogenously administered insulin. Consultation with an endocrinologist is recommended if an alternate cause is considered [42]. The OGTT test should be considered confirmatory for RYGB-related hyperinsulinemic hypoglycemia if glucose levels are less than 55 mg/dL and the patient developed symptoms during testing consistent with their described ambulatory symptoms. Importantly, it should be noted that the OGTT will induce hypoglycemia in up to 12.5 % of control patients and up to 72 % of RYGB patients [6, 43]. Therefore, the lab results should be interpreted with consideration of the clinical presentation of the individual patient.
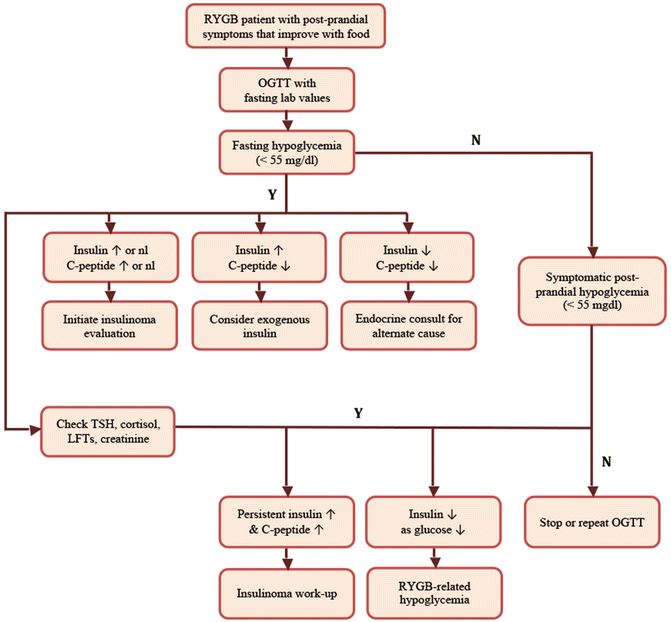

Fig. 21.1
Algorithm for diagnosis of RYGB-related hypoglycemia
Several algorithms for the diagnosis of hypoglycemia involve additional testing to rule out other causes, including insulinoma. It is reasonable to exclude alternate causes of hypoglycemia in RYGB patients when the testing is relatively straightforward and noninvasive. A TSH will screen for hyperthyroidism and an early morning cortisol will screen for adrenal insufficiency. Liver and kidney function should be examined to rule out contributions from severe renal insufficiency or liver disease. However, exclusion of insulinoma is a much more involved process. Insulinoma should be considered if fasting hypoglycemia and hyperinsulinemia are present or if symptoms are not clearly postprandial in nature. However, careful consideration of the expense and the invasiveness of a complete insulinoma rule out should take place if the patient has classic RYGB-related postprandial hyperinsulinemic hypoglycemia symptoms and testing.
Ruling out an insulinoma involves performing a 72-h diagnostic fast. In hyperinsulinemic hypoglycemia, this test should be negative as the hypoglycemia in these patients follows the consumption of food. However, if hypoglycemia and symptoms are not induced with OGTT or a shorter 12 h fast, then a prolonged fast would be indicated. This testing requires inpatient admission and careful coordination to manage the needs for frequent blood draws. The serum glucose, insulin, C-peptide, and proinsulin are measured every 6 h or every 2 h if the glucose level drops below 60 mg/dL. The test is stopped if the patient’s glucose levels drop below 45 mg/dL with the development of neuroglycopenic symptoms. The patient is then tested for serum insulin, C-peptide, proinsulin, beta-hydroxy-butyrate, and sulfonylurea levels. One mg of glucagon is administered and the serum glucose levels are checked at 10, 20 and 30 min [44].
A computed tomography scan of the abdomen and pelvis should be obtained to evaluate for masses in the pancreas if fasting or persistent hyperinsulinemia with hypoglycemia is seen. Some surgeons will also evaluate the pancreas with endoscopic ultrasound. If there is high suspicion, calcium-stimulated arterial angiography can be used to identify more precisely the location of an insulin-secreting pancreatic lesion.
21.4 Management
21.4.1 Diet Modification
Dietary modification is extremely effective and should be the mainstay of therapy for most patients with RYGB-related postprandial hyperinsulinemic hypoglycemia [4, 45]. Most patients naturally alter their own diets over time to avoid the unpleasant symptoms associated with this syndrome. Patients should be encouraged to eat a low-carbohydrate, high-protein diet with appropriate amounts of complex carbohydrates and fat for calories [1, 46]. Simple sugars such as candy or soda should be avoided [1]. Eating smaller, more frequent meals can also help to alleviate symptoms [1, 4, 46], but this must still be combined with a low carbohydrate intake or the patient will just have recurrent symptoms throughout the day. Lying supine for 30 min after a meal can minimize symptoms of dizziness and syncope [1, 46].
21.4.2 Continuous Glucose Monitoring Therapy
With recurrent hypoglycemia associated with hypoglycemia unawareness, consultation with an endocrinologist is recommended to consider use of a continuous glucose monitoring system (CGMS). With this technology, patients will have warning of hypoglycemia and can treat appropriately before severe cognitive impairment.
21.4.3 Pharmacologic Therapy
Approximately 3–5 % of patients will have more severe symptoms of hyperinsulinemic hypoglycemia that will not resolve with dietary modification alone [1]. A variety of pharmacologic agents have been used to alleviate refractory symptoms.
Alpha-glucosidase inhibitors, such as acarbose, were first shown to ameliorate the symptoms of dumping syndrome in 1979 [47]. The drug serves to slow the absorption of glucose from the small intestine, reducing the direct stimulation of the pancreatic beta-cells due to acute hyperglycemia. A randomized, double-blind trial showed significantly lower peak plasma glucose, insulin, and gastric inhibitory polypeptide (GIP) levels when compared with a placebo [47]. Therapeutic dosing ranges from 50 to 100 mg administered two or three times daily 30 min prior to a meal. Acarbose has also been shown to be effective in treating severe hyperinsulinemic hypoglycemia in many reports [48–52]; however, others have noted limited improvement in symptoms [1]. The use of acarbose may be limited by diarrhea and flatulence [1].
Diazoxide was first developed as an antihypertensive medication, but was found to inhibit insulin secretion from pancreatic beta-cells. It does this by opening the ATP-sensitive K+ channels causing hyperpolarization and ultimately eliminating the influx of Ca2+ thus stopping the secretion of insulin. The inhibition of insulin also leads to an increase in glucose production from the liver [49]. One case reports describes a patient with RYGB-related hyperinsulinemic hypoglycemia who failed both surgical therapy with a subtotal pancreatectomy, as well as medical therapy with octreotide, voglibose, and diet modification, but had successful treatment of severe nocturnal hypoglycemia with administration of diazoxide [49]. Side effects can include facial flushing, edema, and weight increase. Diazoxide administration also can lead to hyperglycemia.
Somatostatin is a peptide hormone secreted by the gastric antrum, duodenum, and pancreas which inhibits the release of many other gastrointestinal hormones, including insulin and glucagon. Additionally, it reduces the rate of gastric emptying, slows intestinal transit time, as well as reducing motility, absorption of nutrients, and splanchnic blood flow [46]. In one randomized, double-blind trial comparing the somatostatin analogue, octreotide acetate, to placebo, it was found to completely prevent the development of both vasomotor and gastrointestinal symptoms of “dumping syndrome” [53]. Overall, five randomized, controlled trials have been conducted confirming the efficacy of octreotide [46]. The dosing is 50–100 μg administered subcutaneously three times a day, 30 min prior to a meal. Side effects can be significant and include nausea, abdominal pain, flatulence and diarrhea. Long-term administration of octreotide is associated with gall bladder dysfunction and increased risk of diabetes. Octreotide can be highly effective in preventing the symptoms of both early and late “dumping syndrome” in over 90 % of patients [1, 46]. Somatostatin analogues have also shown efficacy in reducing postprandial hyperinsulinemic hypoglycemia in recent case reports [54, 55].
21.4.4 Endoluminal Therapies
Some providers have attributed “intractable dumping syndrome,” which clinically may appear indistinguishable from postprandial hyperinsulinemic hypoglycemia, to rapid emptying of the gastric pouch through a dilated gastrojejunal anastomosis. They have proposed an endoscopic tightening procedure to delay emptying of the gastric pouch in an effort to alleviate symptoms [57]. The endoscopic procedure begins with measuring the gastrojejunal anastomosis as well as the pouch size. The mucosa of the anastomosis is ablated using an argon plasma coagulator. The EndoCinch suturing system (CR Bard, Murray Hill, NJ) is then used to place endoscopic sutures to plicate the anterior and posterior aspects of the anastomotic ring together with a goal anastomotic lumen of less than 1 cm. Fibrin glue is then applied to the sutures areas. All six patients in this series reported complete resolution of symptoms lasting for a median follow-up of over 600 days. The limitations of this study, however, include a lack of documentation of postprandial hyperinsulinemic hypoglycemia and subsequent objective resolution as all patient results are obtained from clinical interview only.
Another group used the StomaphyX device (EndoGastric Solutions, Redmond, WA) to plicate the gastrojejunostomy in 42 patients with “severe dumping syndrome” [58]. This device places 3–0 polypropylene “H” fasteners circumferentially at 1–2 cm intervals from just proximal to the anastomosis to the gastroesophageal junction in order to cinch down the anastomosis as well as the size of the pouch. They report complete resolution of symptoms in 71 % of patients and improvement of symptoms in all patients. Again, the limitations of this study are related to their definition of “dumping syndrome” and preoperative and postoperative documentation of objective factors. It is unclear whether these two studies can be applied to patients with RYGB-related hyperinsulinemic hypoglycemia.
21.4.5 Pancreatectomy
Pancreatectomy has been described by various authors as a potentially curative surgical intervention for RYGB-related hyperinsulinemic hypoglycemia for the past 20 years; however, as described above, there are many complicating factors which make this technique a poor candidate for appropriate therapy. Multiple case series have been reported of patient with postprandial hyperinsulinemic hypoglycemia after RYGB who were then treated with subtotal or distal pancreatectomy [23–26]. Unfortunately, in all three series, most of the treated patients had no resolution of symptoms or had recurrence of symptomatic hypoglycemia at 1 year [23–25]. At least 25 percent of patients in another study experienced zero benefit from partial pancreatectomy [59]. The overall recurrence of symptoms after partial pancreatectomy has been reported as high as 87 % with a median time to recurrence of 16 months [59]. Therefore, in some cases, the patients ultimately underwent total pancreatectomy to resolve symptoms, but resulting in brittle diabetes.
It seems clear then that pancreatectomy neither addresses the underlying pathophysiology leading to hypoglycemia nor leads to resolution of symptoms in most patients. Additionally, sub-total or total pancreatectomy has elevated perioperative morbidity and may lead to brittle diabetes and other dysfunction related to extensive or complete pancreatic resection. Thus, pancreatectomy should not be offered as therapeutic option in these patients.
21.4.6 Reversal of Gastric Bypass
Reversal of a RYGB to normal anatomy was first described in 2006 [60]. Multiple groups had previously described the conversion of RYGB to other bariatric procedures and documented the feasibility of performing revisions laparoscopically. The indication for this first reversal was incapacitating “dumping syndrome” and the patient had complete resolution of symptoms in addition to maintaining her pre-reversal weight loss.
Postprandial hyperinsulinemic hypoglycemia refractory to diet modification or medical therapy is an indication for reversal of gastric bypass to either normal anatomy or modified sleeve gastrectomy. Preoperative evaluation as described above should be performed to confirm the diagnosis. An esophagogastroduodenoscopy can be performed to confirm pouch size and anatomy and to rule out other pathology. The decision to reverse to normal anatomy or a modified sleeve gastrectomy is complex and based on individual patient characteristics including prior history of gastroesophageal reflux before RYGB.
Prior to consideration of a reversal procedure, we recommend laparoscopic placement of a gastrostomy tube in the gastric remnant followed by a Meal Tolerance Test (MTT) through the excluded gastric route and through the RYGB anatomy to document normalization of glucose and GI and pancreatic hormones postprandial kinetics [40]. The patient is also encouraged to use the gastrostomy tube for feedings and this allows for confirmation that the patient’s postprandial hypoglycemia symptoms will resolve upon surgical reversal. Feeding via a gastrostomy tube prior to surgery can also help avoid refeeding syndrome in cases where the patient is malnourished or has had significant weight loss [61].
The technique has been described in detail previously [40]. The patient is placed in the modified lithotomy position after general anesthesia with an endotracheal tube has been obtained. The abdomen can be entered using either direct trocar insertion with or without Veress insufflation or with the Hasson technique. Five or six trocars should be placed at the surgeon’s discretion to aid in dissection.
The procedure begins with dissection of adhesions around the alimentary limb, gastric pouch and gastric remnant. The gastrojejunostomy is identified and dissected circumferentially. The alimentary limb and the common channel should be measured for length estimates. A linear stapler is fired across the gastric pouch just proximal to the gastrojejunal anastomosis (Fig. 21.2). Care should be taken to avoid injuring the left gastric artery as it is the only blood supply to the gastric pouch. In general, dissection should be kept to a minimum to avoid complications.
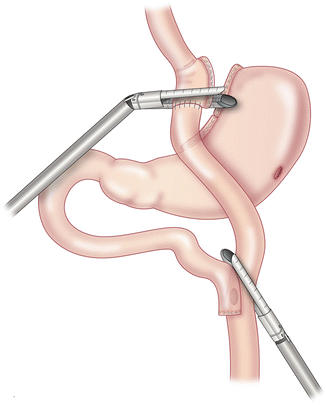

Fig. 21.2




Division of gastrojejunostomy and alimentary limb using linear staplers. With permission from Campos GM, Ziemelis M, Paparodis R, Ahmed M, Davis DB. Laparoscopic reversal of Roux-en-Y gastric bypass: technique and utility for treatment of endocrine complications. Surg Obes Relat Dis. 2014;10(1):36–43. doi:10.1016/j.soard.2013.05.012. Epub 2013 Jun 29 [95]. © Elsevier
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







