Fig. 2.1
American Society of Anesthesiologists Difficult Airway Algorithm [64] © Wolters Kluwer with permission
2.3 Intubation
The ASA recommends components of a preoperative airway physical exam [7]. Table 2.1 displays some findings of the airway physical examination that may suggest the presence of a difficult intubation. BMI alone is not a predictor of difficult intubation [8]. A Mallampati score of III or IV and large neck circumference increase the potential for difficult intubation [9]. Limited jaw protrusion is also a predictor of difficult intubation [2]. Assessment of Mallampati classification when the patient’s craniocervical junction is extended gives a better predictive value for difficult intubation in the morbidly obese population [10].
Airway examination component | Nonreassuring findings |
|---|---|
Length of upper incisors | Relatively long |
Relationship of maxillary and mandibular incisors | Prominent “overbite” (maxillary incisors anterior to mandibular incisors) |
Relationship of maxillary and mandibular incisors during voluntary protrusion of mandible | Patient cannot bring mandibular incisors anterior to maxillary incisors |
Interincisor distance | Less than 3 cm |
Visibility of uvula | Not visible when tongue is protruded with patient in sitting position (e.g., Mallampati class >2) |
Shape of palate | Highly arched or very narrow |
Compliance of mandibular space | Stiff, indurated, occupied by mass, or nonresilient |
Thyromental distance | Less than three ordinary finger breadths |
Length of neck | Short |
Thickness of neck | Thick |
Range of motion of head and neck | Patient cannot touch tip of chin to chest or cannot extend neck |
2.4 Optimizing Position and Preoxygenation
Obese patients have decreased functional residual capacity (FRC), reduced oxygen supply, and increased risk of developing hypoxemia when apneic [11, 12]. Preoxygenation in reverse Trendelenburg (head up) position extends the period prior to desaturation, allowing more time for intubation and airway control [13, 14]. Reverse Trendelenburg position also improves intraoperative oxygenation if appropriate from a surgical perspective [15]. A footboard will prevent the patient from sliding downward while in reverse Trendelenburg position. In addition, sniffing position can facilitate tracheal intubation [16].
Preoxygenation with 100 % O2 either with 3-min tidal volume breathing or four vital capacity breaths provides equal and adequate arterial oxygenation for rapid sequence induction and intubation in the morbidly obese patient [17]. However, Gambee et al. [18] found apneic patients developed desaturation more rapidly after preoxygenation with four vital capacity breaths than did patients preoxygenated for 3 min. Thus tidal volume breathing for 3–5 min or eight vital capacity breathing over 60 s is recommended. The goal of preoxygenation is to achieve an end-tidal oxygenation concentration >90 %.
Application of continuous positive airway pressure (CPAP) of 10 cm H2O during preoxygenation and positive end-expiratory pressure (PEEP) of 10 cm H2O during mask ventilation after the induction of anesthesia can reduce atelectasis formation in morbidly obese patients and improve oxygenation [19]. This is a useful technique to prolong time to desaturation [20].
2.5 Laryngeal Mask Airway
The laryngeal mask airway (LMA) is an important element of the difficult airway management algorithm [7] as a rescue option if face mask ventilation is not adequate. Even though most anesthesiologists feel the LMA is not appropriate to use as a definitive airway during bariatric surgery due to aspiration risk, the intubating LMA serves as an effective conduit for intubation. Frappier et al. used the intubating LMA in morbidly obese patients with a 96.3 % success rate [21]. Patients requiring rapid sequence induction were excluded from this study.
2.6 Choice of Laryngoscope
Choices for laryngoscopy include direct laryngoscopy with standard Macintosh or Miller blades, video laryngoscopes such as the Glidescope®, and flexible fiber-optic bronchoscopes. Selection among those devices is based on preoperative airway assessment. Video laryngoscopy improves intubation conditions in the morbidly obese patient [22]. If a non-video laryngoscope is chosen as the first line equipment, it is prudent to have a video laryngoscope readily available as backup.
Awake flexible fiber-optic laryngoscopy remains the gold standard for managing difficult airways. The patient with a history of failed intubation, upper airway abnormality, or with an expected difficult intubation from another cause may benefit from an awake fiber-optic intubation. Awake fiber-optic intubation requires an experienced operator. Proper topical anesthesia and careful sedation is key for success.
2.7 Extubation
A thorough evaluation of readiness for extubation must be performed at the conclusion of the anesthetic. There should be no ongoing indication to keep the patient intubated, such as hemodynamic instability. For patients who are difficult to intubate, proper timing of extubation, equipment availability, and the presence of skilled anesthesia providers are all vital for safe extubation. Before extubation, the following criteria should be met: spontaneous ventilation must be adequate, muscle relaxant fully reversed, airway reflexes fully recovered and the patient is following commands. If the intubation was difficult, the presence of a cuff leak should be documented prior to extubation to rule out glottic swelling due to airway trauma. To increase FRC before extubation, the patient should be placed in the reverse Trendelenburg position. For patients with OSA, extubation directly to noninvasive positive pressure ventilation may reduce airway obstruction and improve respiratory function [23].
2.8 Intraoperative Management
2.8.1 Intraoperative Monitoring
Standard intraoperative anesthesia monitoring requires continuous evaluation of oxygenation, ventilation, circulation, and temperature. Pulse oximetry is used for the monitoring of oxygenation during the perioperative period and should be maintained as long as patients remain at increased risk for airway compromise [24]. Capnography is used to monitor ventilation intraoperatively. End-tidal carbon dioxide (ETCO2) may not accurately reflect arterial carbon dioxide tension (PaCO2) due to ventilation–perfusion mismatch in severely obese patients. Transcutaneous CO2 (TcCO2) monitoring may provide an alternative to end-tidal carbon dioxide monitoring, and has been shown to be more accurate than ETCO2 at estimating PaCO2 [25, 26].
Choosing a properly sized blood pressure cuff is important for accurate blood pressure measurement. The blood pressure cuff bladder width and length should be approximately 40 % and 80 % of the upper arm circumference, respectively. Blood pressure may be measured on the forearm if the upper arm is not anatomically amenable to blood pressure cuff placement. The decision to place an invasive monitor such as an arterial or central venous catheter should be guided by the clinical circumstances and comorbidities such as cerebral vascular disease, coronary artery disease, or pulmonary hypertension.
Obese patients are at increased risk for postoperative respiratory complications. Full reversal of neuromuscular blockade should be verified via both qualitative and quantitative measures. Instead of conventional qualitative train-of-four monitoring, intraoperative acceleromyography monitoring reduces the incidence of residual blockade and respiratory complications in the post anesthesia care unit [27].
Hypothermia has been associated with several perioperative complications, including wound infection, cardiac events, immune dysfunction, coagulopathy, and increased blood loss [28]. Body temperature should be measured during all bariatric operations. Forced air warming can be used to maintain normothermia intraoperatively.
2.8.2 Intraoperative Ventilation
Futier et al. demonstrated that using intraoperative low tidal volume lung protective ventilation in abdominal surgery reduced 7-day postoperative respiratory complications when compared with nonprotective ventilation [29]. The lung protective ventilation group used a tidal volume of 6–8 ml per kilogram of predicted body weight, PEEP of 6–8 cmH2O, and recruitment maneuvers every 30 min. Chalhoub et al. used recruitment maneuvers following by PEEP in morbidly obese patients undergoing bariatric surgery and showed improvement in arterial oxygenation [30]. Erlandsson et al. used electric impedance tomography to optimize PEEP in morbidly obese patients. The PEEP level determined to prevent lung collapse and to improve gas exchange in morbidly obese patients in this study was around 15 cm H2O. Based on current literature, low tidal volume based on predicted body weight, PEEP, and recruitment maneuvers are the three components to optimize intraoperative ventilation. Adjustments should be based on an assessment of ETCO2, oxygenation, hemodynamics, and patient volume status.
2.8.3 Intraoperative Positioning and Preventing Nerve Injury
Appropriate positioning of the morbidly obese patient is important for both the patient and the operating room staff. Attention should be paid to the weight limit of the operating table as the maximum allowable weight may vary dependent upon the orientation of the table. It may be necessary to use table extenders that attach to the siderails to accommodate the largest patients. Commercially available devices such as the HoverMatt® (HoverTech International, Bethlehem, PA) can be used to facilitate transfer of the obese patient between the operating room table and hospital bed. It is also critical to have adequate personnel to assist in moving and positioning morbidly obese patients. To minimize the risk of fall from the operating room table, straps and a footboard should be used for security. The use of a footboard is helpful to avoid downward sliding with reverse Trendelenburg position during induction, surgery (if indicated), and emergence. Given the higher risk of nerve injury in the obese population, all pressure points need to be adequately padded during surgery. Padding may include gel pads, foam, air-filled pads, or other padding materials.
2.9 Perioperative Drug Dosing
Identifying the appropriate dose of anesthetic medications can be challenging with obese patients. For the morbidly obese patient, dosing based on actual body weight overestimates requirements for fentanyl [31], cisatracurium [32], and rocuronium [33]. Different agents are dosed based on different body weight scalars such as total body weight (TBW), lean body weight (LBW), or ideal body weight (IBW), depending on their pharmacokinetic characteristics. Different phases of anesthesia, such as induction and maintenance, may require using different scalars in dosing calculations as well. For medications with limited pharmacokinetic data, anesthesiologists can begin dosing closer to the patient’s estimated lean body mass (about 120 % of ideal body weight) and adjust as needed [34] (Tables 2.2 and 2.3).
Table 2.2
Body weight calculations
Body weight | Formula |
|---|---|
Ideal body weight (IBW) [65] | Male: IBW = 50 kg + 2.3 kg/each inch above 5 feet Female: IBW = 50 kg + 2.3 kg/each inch above 5 feet |
Lean body weight (LBW) [66] | Male: LBW = 0.33 × weight (kg) + 0.34 × height (cm)−29.53 Female: LBW = 0.30 × weight (kg) + 0.42 × height (cm)−43.30 |
Total body weight (TBW) | The patient actual body weight |
Drug | Dosing scalar |
|---|---|
Thiopental | Induction: LBM Maintenance: TBW |
Propofol | Induction: LBM Maintenance: TBW |
Fentanyl | LBM |
Remifentanil | LBM |
Succinylcholine | TBW |
Vecuronium | IBW |
Rocuronium | IBW |
Cisatracurium | IBW |
2.10 Perioperative Pain Management
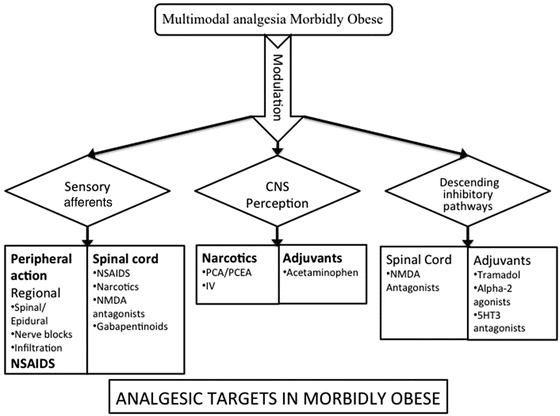
Because of respiratory depression and other side effects associated with opioids, multimodal pain management with minimized opioid use may be a better strategy in morbidly obese patients undergoing bariatric surgery [35]. A multimodal regimen works at different targets, from central to peripheral levels (Fig. 2.2). The use of multiple drugs, analgesic or adjuvant, in combination with opioids, achieves the best pain relief in obese patients during their postoperative course while minimizing side effects of opioids. Nonopioid analgesic options include NSAIDs, acetaminophen, alpha-2 agonists, NMDA receptor antagonists, magnesium, and neuropathic pain medications such as pregabalin or gabapentin. Feld et al. [36] used a nonopioid regimen including ketorolac, clonidine, lidocaine, ketamine, magnesium sulfate, and methylprednisolone. Nonopioid treated patients required less supplemental morphine PCA use and were also less sedated. However, more systematic studies are required before we can recommend specific protocols of multimodal pain management for bariatric surgery patients (see Fig. 2.2).
Local anesthetics via various delivery methods such as transversus abdominis plane (TAP) block or surgical site infusion can significantly reduce incisional pain. Spinal or epidural analgesia provides excellent pain control for patients undergoing open bariatric surgery.
2.11 Nonopioid Analgesics
2.11.1 NSAIDs
Both selective and non-selective cyclooxygenase II (COX-II) inhibitors may be used in multimodal pain regimens. Govindarajan et al. [37] demonstrated a significant reduction in narcotic requirements with ketorolac during the first 24-h postoperatively in morbidly obese patients undergoing laparoscopic surgery. Ketorolac provides comparable postoperative pain relief to fentanyl while lowering the incidence of nausea and sedation [38]. Gastric perforation has been reported in the morbidly obese with prolonged use of non-selective COX inhibitors. Cox-II inhibitors have been advocated if long-term analgesics are necessary [39]. NSAIDs are most successfully used as a component of combination therapy rather than sole analgesics in morbidly obese individuals.
2.11.2 Acetaminophen
Acetaminophen is a centrally acting analgesic without sedative effect and with significant opioid sparing ability [40]. Obesity does not seem to alter acetaminophen pharmacokinetics. The dosage of acetaminophen should be based on ideal body weight. Acetaminophen doses at 6-h intervals can be used safely in the absence of other contraindications. A combination of acetaminophen and NSAIDs has been shown to be superior to either single therapy for managing mild to moderate postoperative pain.
2.11.3 Alpha-2 Agonists
Clonidine and dexmedetomidine are alpha-2 agonists with analgesic properties. Dexmedetomidine is more effective than clonidine in analgesia due to its increased selectivity for alpha-2A receptors. Dexmedetomidine can maintain airway tone and respiratory drive, making it is a good choice in morbidly obese patients. It lowers intraoperative analgesic requirements and suppresses postoperative nausea vomiting (PONV) when run as an infusion at 0.2–0.8 mcg/kg/min [41]. Clonidine and dexmedetomidine can decrease postoperative rescue analgesic requirements by 25 % and 30 %, respectively, in morbidly obese patients [42].
2.11.4 Ketamine
Ketamine is a N-methyl-D-aspartate (NMDA) antagonist, which prevents glutamate action in pain transmission. It also reverses opioid induced hyperalgesia. Ketamine used as an analgesic adjuvant can reduce opioid use [36]. Hallucinatory side-effects can be avoided with a 0.5 mg/kg bolus dose followed by a continuous infusion of 2.0–2.5 mcg/kg/min for the first 48 h postoperatively [43].
2.11.5 Magnesium
Magnesium, by blocking NMDA receptors (at sites other than those blocked by ketamine) is known to reduce both intraoperative and postoperative analgesic requirements. Ryu et al. [44] added magnesium at a dose of 50 mg/kg at the time of induction and reported improved quality of postoperative analgesia in gynecological surgery cases.
2.11.6 Pregabalin and Gabapentin
Pregabalin and gabapentin inhibit calcium currents via high-voltage-activated channels, reducing neurotransmitter release and attenuating postsynaptic excitability. They are successfully used for chronic pain treatment. A large number of clinical trials indicate that pregabalin and gabapentin can be effective as postoperative analgesics. One oral dose of pregabalin 150 mg 2 h before laparoscopic sleeve gastrectomy reduced 24-h morphine use postoperatively [45]. One dose of gabapentin 600 mg 1 h before general anesthesia reduced opioid consumption in various surgeries [46]. Because of the sedative effect, pregabalin and gabapentin should be used cautiously in elderly patients. Both are eliminated solely by renal clearance and dosage must be adjusted in renally impaired patients.
2.11.7 Regional Blocks
The transversus abdominis plane (TAP) block is particularly effective in blocking T10 to L1 segments and is an attractive option for laparoscopic or open abdominal surgery. Bilateral TAP block in the nonobese patient has shown efficacy with midline incisions [47]. It can be used as a rescue option in situations of failed/difficult epidural analgesia in open bariatric surgery patients. Ultrasound guidance may reduce the challenge of performing TAP block in morbidly obese patients.
Infusion of local anesthetic at the surgical wound site is another convenient analgesia option for bariatric surgery. Both continuous flow devices and patient controlled pumps can be used. A bupivacaine pump has been found to reduce the use of opioids in morbidly obese undergoing laparoscopic bariatric surgery although its use is somewhat controversial [48].
Infusion of intraperitoneal 0.375 % bupivacaine for morbidly obese patients undergoing laparoscopic gastric banding showed significant pain score reduction postoperatively [49]. In a retrospective review, postoperative morphine use after Roux-en-Y bypass surgery was significantly lower in the bupivacaine intraperitoneal group than the control group [50].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree