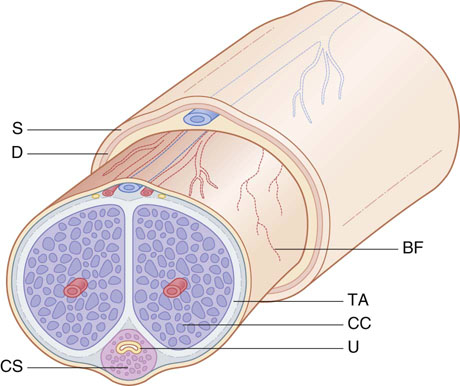
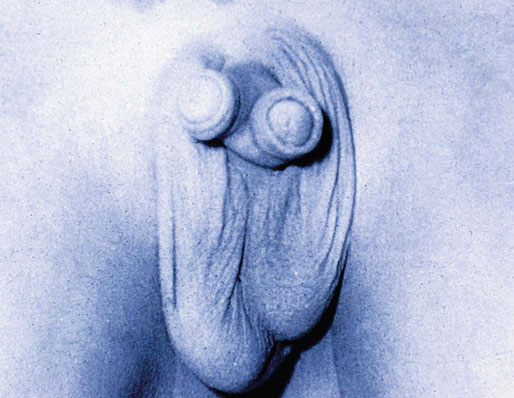
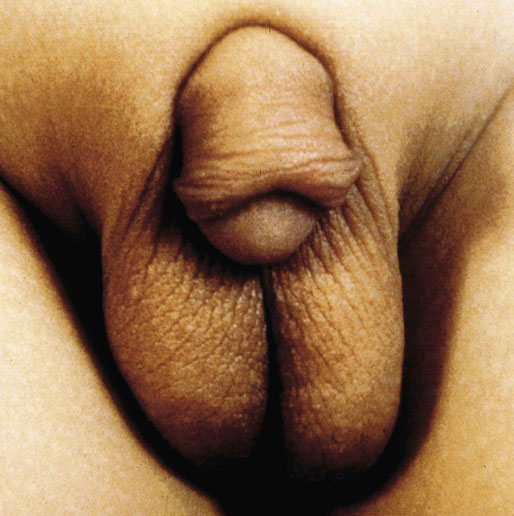
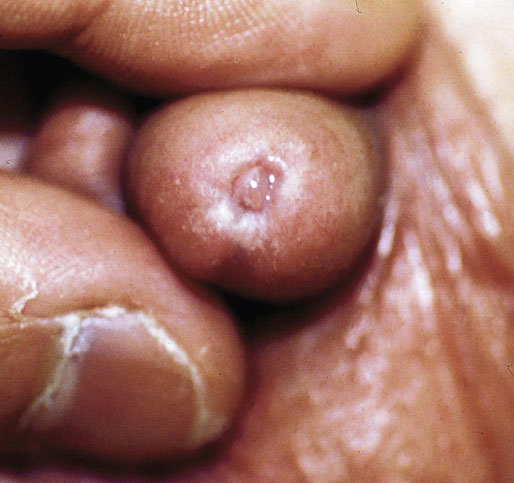
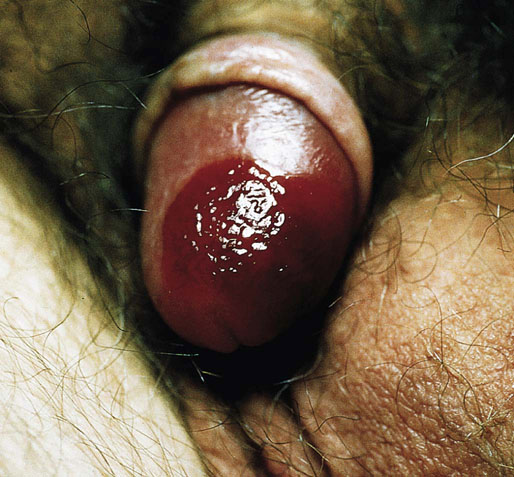
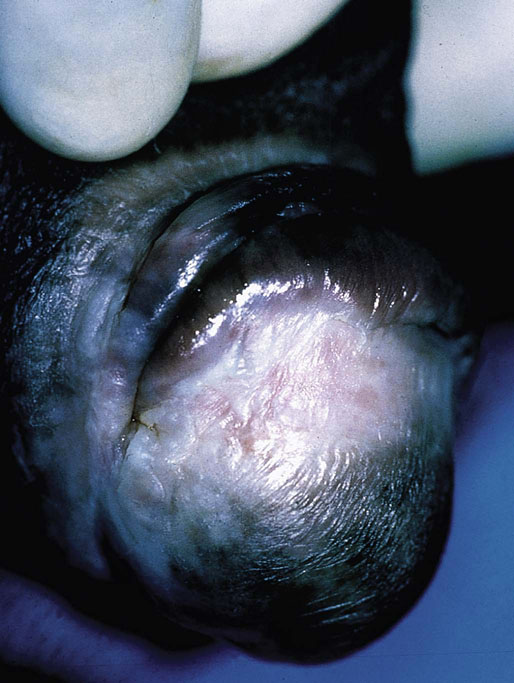
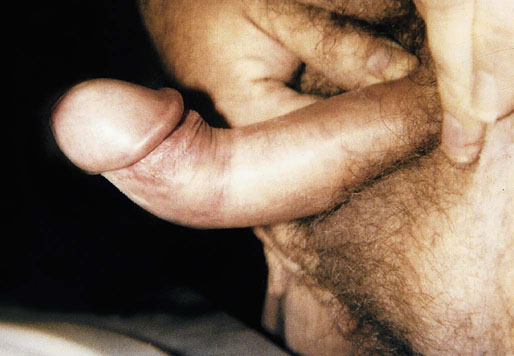
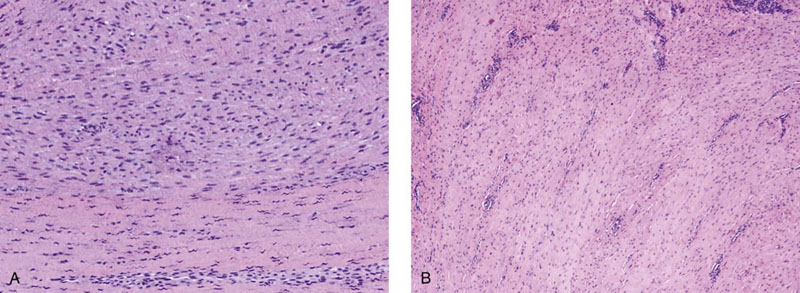
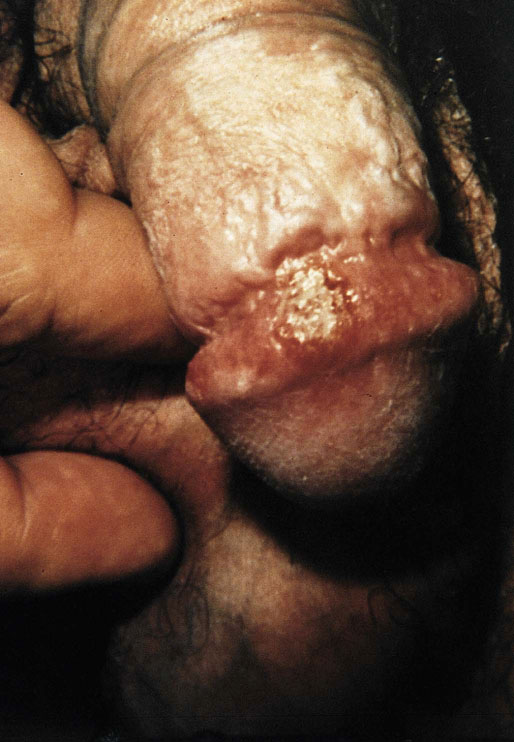
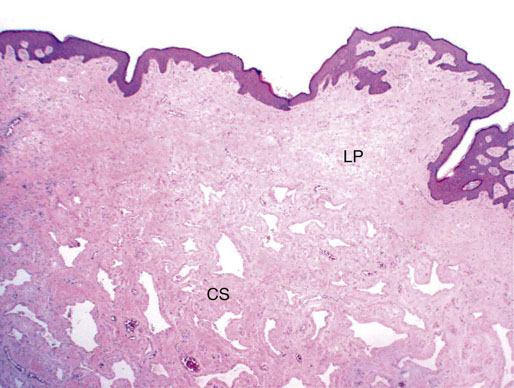
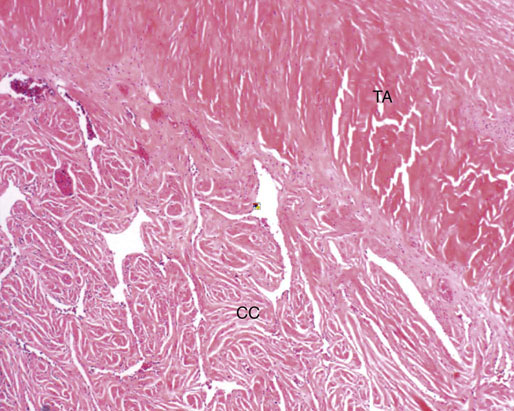
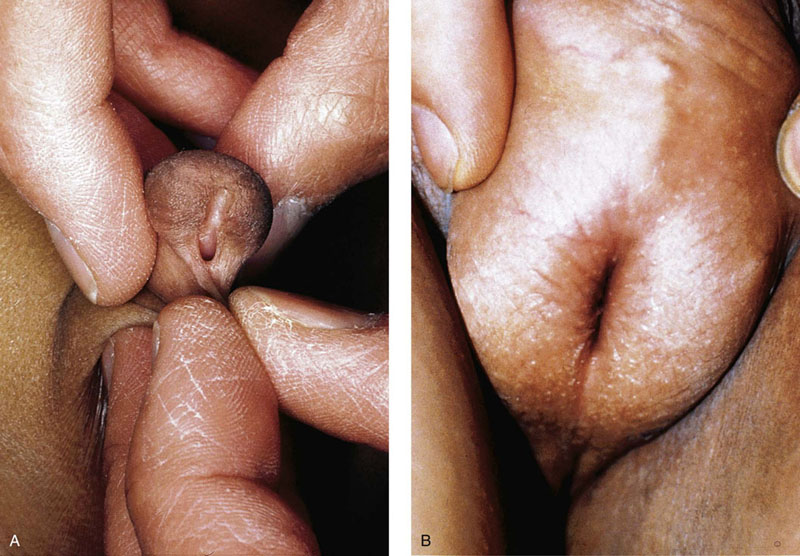
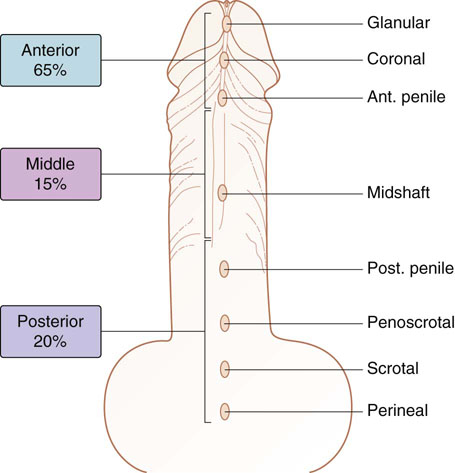
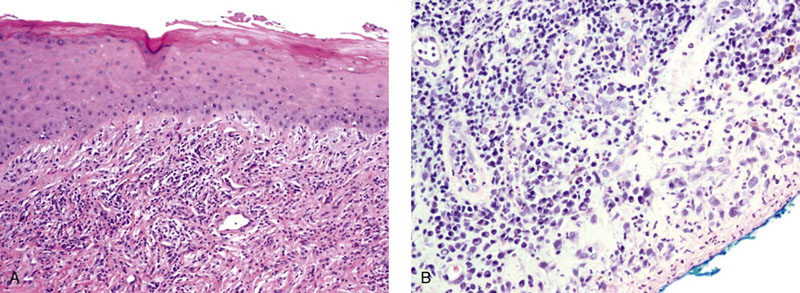
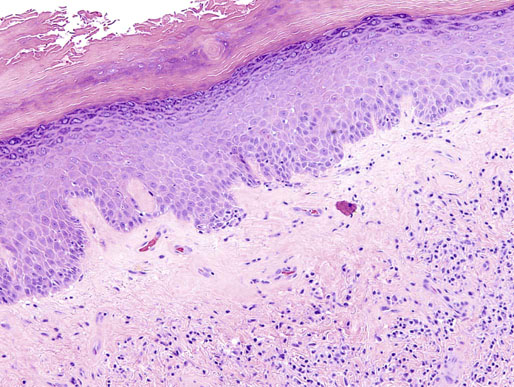
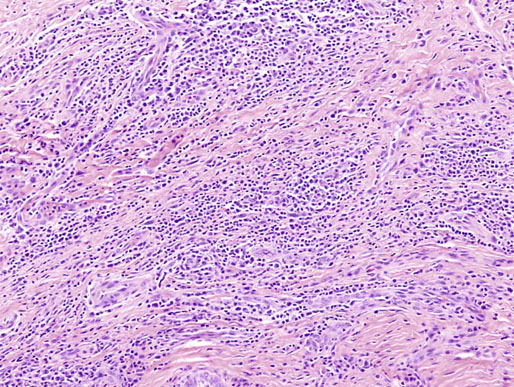
Chapter 15 Histologically, the foreskin comprises five layers: epidermis, dermis, dartos muscle, lamina propria, and squamous mucosa. The squamous mucosa of the foreskin is a prolongation of that of the glans and balanopreputial sulcus. Langerhans cells are present in the mucosal epithelium of the foreskin and are more numerous in the foreskin than those identified in the female cervical tissue.1 The foreskin is highly vascular. Most of its blood supply arises from the internal pudendal artery, which has three main branches: the deep artery, the bulbar artery, and the urethral artery. The venous return is through three channels: the cavernous veins, the deep veins, and the superficial dorsal veins. The lymphatic drainage is through the superficial and deep inguinal lymph nodes that drain to the external and common iliac nodes. The corpus spongiosum is the main structure of the glans; it consists of an 8- to 10-mm thick layer of highly vascularized erectile tissue with varying-sized vessels, smooth muscle fibers, and peripheral nerves. The transition between the lamina propria and corpus spongiosum is usually not well delineated and often difficult to determine (Fig. 15-1). The corpora cavernosa form the main component of the shaft of penis and extend into the glans to a variable degree among males. The corpus spongiosum is separated from the corpora cavernosa by a dense, white, fibroelastic membrane—tunica albuginea (Fig. 15-2). It is 1 to 2 mm thick in the flaccid state, but becomes thinner during erection, and serves as an important barrier to the spread of cancer to the corpora cavernosa. Fig. 15-1 Glans penis with lamina propria (LP) and corpus spongiosum (CS) showing venous channels with fibromuscular stroma. Fig. 15-2 Tunica albuginea (TA) and corpus cavernosum (CC) showing erectile tissue similar to corpus spongiosum, with a more muscular appearance. The coronal sulcus between the glans and the shaft is a narrow and circumferential cul-de-sac located just below the corona of the penis. The sulcus is composed of squamous mucosa, lamina propria, dartos muscle, and Buck fascia and is a common site for recurrence of carcinoma or a positive margin in cases of primary foreskin carcinoma.2 The body or shaft of the penis is composed of (1) a thin, wrinkled, pigmented epidermis with few adnexal structures; (2) dermis; (3) dartos muscle; (4) adipose tissue; (5) Buck fascia with numerous vessels and nerves; (6) tunica albuginea; and (7) erectile tissue of corpora cavernosa and corpus spongiosum, the latter encasing the urethra (Fig. 15-3). Histogenetically, the penis has two separate origins for its three erectile bodies. The corpora cavernosa are derived from the genital tubercles, whereas the urethra and corpus spongiosum are formed from the urogenital sinus and the urogenital folds. A comprehensive list of penile lesions is included in Table 15-1. Table 15-1 AIDS, Acquired immunodeficiency syndrome. *Carcinosarcoma is included with sarcomatoid carcinoma for purposes of this work. These tumors are also designated metaplastic carcinoma by some authors. Congenital absence of the upper wall of the urethra is known as epispadias. In this anomaly, the urethral opening is situated on the dorsum of the penis as a groove or cleft. The incidence of epispadias is 1 in 117,000 male births.3,4 According to the location, the three types of epispadias are penopubic, penile, and glanular, with penopubic being most frequent.3 Urinary incontinence is frequently observed with penopubic epispadias and occasionally with penile type, but it is not associated with glanular epispadias.4 Associated congenital anomalies include diastasis of the pubic symphysis, bladder exstrophy, renal agenesis, and ectopic pelvic kidney.4–11 Hypospadias is a developmental anomaly in which the urethra opens on the underside of the penile shaft or on the perineum (Fig. 15-4).12–14 Hypospadias is frequently associated with chordee (Fig. 15-5) but also can be seen as an independent finding. Hypospadias is classified based on the location of the meatus (glanular, subcoronal, distal penile, midshaft, proximal penile, penoscrotal, scrotal, or perineal) (Fig. 15-6). The glanular and subcoronal types constitute anterior hypospadias (see Fig. 15-4, A), which accounts for 50% of all cases. Distal penile, midshaft, and proximal types make up middle hypospadias, accounting for 30% of patients. The remaining 20% are posterior hypospadias and include penoscrotal, scrotal (see Fig. 15-4, B), and perineal types.12,13 The incidence of hypospadias is 1 per 300 live male births.13,15 Associated anomalies include cryptorchidism and inguinal hernia. Association with anomalies of the upper urinary tract is uncommon unless other anomalies are present in other organ systems.13–17 Fig. 15-4 A, Distal hypospadias with the urethral meatus at the junction of the glans and penile shaft. B, Proximal hypospadias with the urethral meatus at the base of scrotum. (Courtesy Dr. Hyum Yul Rhew, Kosin University, Busan, Korea.) Fig. 15-6 Location of hypospadias. Ant, Anterior; Post, posterior. (From Mr. Subhendu Chakraborty, The Methodist Hospital, Houston, Tex.) Micropenis is a normally formed penis with a size 2 or more standard deviations below the mean. The ratio of the length of the penile shaft to its circumference is normal (Fig. 15-7). The corpora cavernosa may be severely hypoplastic. The scrotum is generally fused but often diminutive, and the testes usually are small and frequently cryptorchid. A webbed or concealed penis often resembles a micropenis, but the penile shaft is of normal length. The three most common causes of micropenis are hypogonadotropic hypogonadism, hypergonadotropic hypogonadism (primary testicular failure), and idiopathic.18–20 Concealed penis is a normally developed penis that becomes buried in a suprapubic fat pad (Fig. 15-8). This anomaly may be congenital or idiopathic after circumcision. A concealed penis may be visualized by retracting skin lateral to the penile shaft.21 Penoplasty alone or penoplasty with liposuction of prominent prepubic fat pad used to correct concealed penis alleviates the initial complaint and provides good cosmetic and functional results with greater satisfaction in older patients.22 Aphallia (penile agenesis) results from failure of the genital tubercle to develop. The incidence is 1 in 10,000,000 live male births; only 70 cases have been reported.23,24 The usual appearance is that of a well-developed scrotum with descended testes but no penile shaft. In most cases, the urethra opens at the anal verge adjacent to a small skin tag or, in other cases, opens into the rectum. Gender reassignment is of prime significance in such cases, necessitating immediate clinical assessment. Associated malformations include cryptorchidism, vesicoureteral reflux, horseshoe kidney, renal agenesis, imperforate anus, and musculoskeletal and cardiopulmonary abnormalities.23–25 Diphallus, or duplication of the penis, is a rare anomaly that ranges from a small accessory penis to complete duplication (Fig. 15-9).26,27 Associated anomalies include hypospadias, bifid scrotum, duplication of the bladder, renal agenesis or ectopia, and diastasis of the pubic symphysis. Anal and cardiac anomalies are also common.26–28 Chordee, a congenital or acquired bend of the penis, is caused by decreased elasticity in one or more of the fascial layers of the penis, leading to shortness of one corpus cavernosum when erection occurs. The bend may be ventral, dorsal, lateral, or complex. Chordee is most frequently associated developmentally with hypospadias when the mesenchyme distal to the meatus ceases to differentiate, creating a fan-shaped band of dysgenetic fascia.29,30 Acquired chordee may result from trauma or Peyronie disease.31 Scrotal engulfment (penoscrotal transposition) results from incomplete migration of the inferomedial labioscrotal swelling (Fig. 15-10). This has been termed bifid scrotum, doughnut scrotum, prepenile scrotum, and shawl scrotum. Frequently it occurs in conjunction with perineal, scrotal, or penoscrotal hypospadias with chordee.32 Ectopic scrotum is rare and refers to the anomalous position of one hemiscrotum along the inguinal canal, most commonly the suprainguinal canal, although it may be within the infrainguinal canal or the perineum. Associated anomalies include cryptorchidism, inguinal hernia, exstrophy, popliteal pterygium syndrome, renal agenesis, renal dysplasia, and ectopic urethra.33 Magnetic resonance imaging (MRI) renders excellent anatomic interpretation of complex genital anomalies and associated abnormal pelvic tissues, thereby assisting surgeons in conceptualizing the anomalous structures and contributing to management.34 After surgical correction of anomalies, long-term follow-up studies have shown that adulthood satisfaction with voiding and sexual function is achieved in approximately two thirds of patients and some degree of dissatisfaction in one third. Epispadias repair is a much more complicated procedure, and long-term results are seldom reported. Nevertheless, good results are achieved with respect to continence (~80% success) and sexual function.35 Phimosis is a condition in which the foreskin cannot be retracted behind the glans penis (Fig. 15-11). In adolescents and adults, the foreskin can normally be retracted beyond the corona with relative ease, but it is important to note that in children younger than 5 years of age the foreskin is not retractable.36 Phimosis may arise in uncircumcised men at any age. Phimosis may be congenital or acquired. Congenital phimosis is much rarer and is secondary to a small preputial orifice or an abnormally long foreskin. Secondary phimosis usually results from an accumulation of smegma, which is due to poor hygiene and can lead to chronic inflammation, edema, and fibrosis. Balanoposthitis (inflammation of the glans and prepuce) and balanitis xerotica obliterans may cause phimosis.36,37 Circumcision is the treatment for phimosis, regardless of etiology. Surgical specimens from men should be carefully examined for areas of induration that might indicate dysplastic or neoplastic lesions.36 Microscopically, phimotic prepuces may be histologically normal or show varying degrees of inflammation, fibrosis, edema, and vascular congestion. Lymphocytes and plasma cells are the predominant inflammatory components.38 Patients with phimosis often complain of irritation, but significant pain is uncommon unless ballooning of the foreskin occurs as a result of urinary obstruction. Paraphimosis is a condition in which the foreskin has been retracted behind the glans penis and cannot be advanced back over the glans.36 Constriction of the glans causes pain from vascular engorgement and edema. Paraphimosis is often iatrogenic, occurring after examination of the penis or after urinary tract instrumentation. Rarer reported causes include Plasmodium falciparum malaria39 and carcinoma metastatic to the penis.40,41 Paraphimosis requires circumcision or emergency dorsal slit surgery.36 Phimosis often coexists with penile carcinoma and is a risk factor for it (see later discussion).42,43 Difficulty in foreskin retraction and phimosis are risk factors for penile carcinoma that may be related to the anatomically variable length of the foreskin. Velazquez and colleagues44 compared foreskin length and status in the general population and patients with penile cancer and found that 77% of men without cancer had a long foreskin and only 7% had phimosis. Patients with cancer had a long foreskin in 78% of patients, and phimosis was significantly increased in frequency (52%). Coexistence of a long foreskin and phimosis may explain the high incidence of penile cancer in some geographic regions. Because phimosis appears to be a major factor, the presence of a long foreskin may be a necessary but not a sufficient condition for cancer development. For these reasons, Velazquez and colleagues supported preventive circumcision in patients with long and phimotic foreskins living in high-risk areas.44 Fibroepithelial polyp is very rare in the penis and usually manifests as a polypoid or cauliflower-like mass or masses involving the glans penis or prepuce.45–48 It ranges in size from less than 1 cm to greater than 7.5 cm in greatest dimension and is strongly associated with long-term condom catheter use, or rarely it may develop in association with phimosis. These lesions are characterized by their large size, and histopathologic examination demonstrates loose, edematous, cellular stroma containing numerous small vessels with luminal dilatation and occasional multinucleated mesenchymal cells; the term lymphedematous fibroepithelial polyp has been assigned to this entity. The age of the patients ranges from 4 to 58 years (median, 40 years) at the time of initial surgical resection, and the preoperative duration varies from 6 months to 10 years. The majority of fibroepithelial polyps affect the ventral surface of the glans near the urethral meatus. Clinically, the differential diagnosis includes cutaneous fibroepithelial polyp (acrochordon), condyloma acuminatum, and even squamous or urothelial carcinoma. Lymphedematous fibroepithelial polyps of the penis are histologically and clinically distinct from the standard acrochordons that are commonly encountered in dermatopathology practice. In contrast to the cutaneous fibroepithelial polyps, lymphedematous fibroepithelial polyps of the penis are typically larger; show stromal hypercellularity with occasional multinucleated cells, increased mast cells, and stromal edema; and have a prominent vascular pattern with dilatation and thickening of vessels. In addition, lymphedematous fibroepithelial polyps have been described only occurring on the penis, whereas the typical locations for cutaneous fibroepithelial polyps are neck, axilla, eyelid, and inframammary folds. The location and clinical appearance raise the clinical impression of condyloma acuminatum; however, no features such as papillomatosis or koilocytosis are seen to suggest this diagnosis. These polyps do not demonstrate any features of a cutaneous or mucosal carcinoma, such as invasion of normal structures, cytologic atypia, or mitoses. The stromal hypercellularity and occasional multinucleation may appear worrisome on low power; however, high-power examination shows bland cytologic morphology. Immunohistochemically, the stromal cells of lymphedematous fibroepithelial polyp demonstrate limited immunoreactivity for muscle-specific actin, α-smooth muscle actin (SMA), and desmin and show no reactivity for S100 protein or CD34.45–48 The precise pathogenesis of these lesions is not known; however, considering the strong association with condom catheter use, they are likely reactive. Treatment with local excision has been successful in all reported cases. Although fibroepithelial polyp may recur, recurrences are also managed in a similar fashion.45 This rare entity may be currently underrecognized or underreported by pathologists or dermatopathologists. In cases in which clinical information regarding the use of a condom catheter may not be readily available, recognizing the distinct histologic features of this lesion with edematous, hypercellular stroma with occasional multinucleated cells and prominent vasculature is important for a correct diagnosis because they will likely be the dermatopathologist’s only diagnostic clues. Balanoposthitis (inflammation of the glans penis and prepuce) and balanitis (inflammation of the glans penis) occur most commonly in uncircumcised men.49–51 The usual cause of balanoposthitis is poor hygiene. Failure to regularly retract and clean the foreskin leads to accumulation of smegma (desquamated epithelial cells and debris), which incites an inflammatory response, and may subsequently result in phimosis. Balanoposthitis also can result from specific dermatologic lesions or infectious agents (Table 15-2). In a series of 86 patients with balanoposthitis and balanitis, 41% had no attributable etiologic factor; Candida species accounted for 30%, and β-hemolytic streptococci were responsible for 11% of patients.52–54 Balanoposthitis caused by Pseudomonas aeruginosa coproducing metallo-β-lactamase and 16S rRNA methylase in children with hematologic malignancies has been reported, although it is rare.53 Candidal balanoposthitis is discussed later, in the section on infectious diseases of the penis. Discussion of papulosquamous and vesiculobullous diseases is beyond the scope of this text. Plasma cell balanitis (Zoon balanitis or balanitis circumscripta plasmacellularis) is a disorder that was first described in 1952 by Zoon.55 The disease is not rare and is important because it clinically resembles squamous cell carcinoma in situ of the glans penis.56,57 Plasma cell balanitis is a benign disorder of unknown etiology that is thought to represent a reaction to a multitude of diverse stimuli. Recently, Houser and colleagues58 reported a case of Zoon balanitis in an African-American man with human immunodeficiency virus (HIV). Plasma cell balanitis affects only uncircumcised males.59 It is similar clinically and histologically to its vulvar counterpart, vulvitis circumscripta plasmacellularis. It usually manifests as a single large (≥2 cm) bright red, moist patch on the glans or inner prepuce (Fig. 15-12). Rarely, multiple patches may be present, and in severe cases it may consist of extensive visibly eroded lesions. The clinical appearance of the lesion overlaps with that of candidal balanitis and squamous cell carcinoma in situ, so biopsy is mandatory. Cases of combined erythroplasia of Queyrat with Zoon balanitis have been reported, and this combination can create a diagnostic dilemma.60 A case of plasma cell balanitis has been reported in a 57-year-old Hispanic man with a remote history of syphilis who presented with a 6-month nonhealing, granulating ulcer of the foreskin and glans penis that had been repeatedly mistaken for syphilis and treated unsuccessfully with circumcision. Biopsy of the glans penis demonstrated denuded chronic granulation tissue showing a fibrotic stroma with numerous blood vessels and a mixed inflammatory infiltrate including scattered plasma cells. It is important to differentiate plasma cell balanitis from a syphilitic chancre in a patient presenting with a nonhealing penile lesion. This case report demonstrates that these entities may be seen in the same patient at different times.61 Histologically, the hallmark of plasma cell balanitis is a distinct upper dermal bandlike infiltrate containing numerous plasma cells (Fig. 15-13).59–64 In some cases, the number of plasma cells may be scant or moderate, and the histologic findings must be correlated with the clinical observations. The dermis also contains numerous dilated capillaries adjacent to extravasated erythrocytes or hemosiderin deposits. The overlying epidermis is thin and may occasionally be absent or partially separated from the dermis. The most distinctive feature within the epidermis is the presence of flattened or diamond- or rhomboid-shaped keratinocytes that are separated by uniform intercellular edema. Fig. 15-13 A, Zoon balanitis with reactive epithelial changes in the epidermis and a dermal inflammatory infiltrate accompanied by edema (low power). B, Zoon balanitis with abundant plasma cells in the inflammatory infiltrate (high power). Mucinous metaplasia of the penis is an uncommon lesion that occurs usually in elderly patients and appears to be a metaplastic change associated with severe chronic inflammation, especially with Zoon balanitis. Mucinous metaplasia may affect the glans penis and the mucosal surface of the foreskin.65,66 Currently, the treatment of choice is circumcision,56,57,63 but laser surgery59,67 and topical application of retin-A preparations, steroids, or tacrolimus (pimecrolimus) have been employed with variable success.68 Lichen sclerosus is a chronic atrophic mucocutaneous condition affecting the epidermis and dermal connective tissue that most commonly involves the genital and perianal skin of both males and females.69 Extragenital lesions may accompany genital lesions, although they also may occur alone.69 Balanitis xerotica obliterans is a term used as a synonym for lichen sclerosus of the glans penis and prepuce.70 This lesion is associated with penile carcinoma, and it has been postulated to be a preneoplastic condition for at least some types of penile cancer, particularly non–human papillomavirus (HPV) variants of squamous cell carcinoma.71–83 Balanitis xerotica obliterans is commonly encountered in preputial resections for phimosis in older men. In contrast, the prepubertal incidence in a series of 117 patients was only 4%.84 The idiopathic form of balanitis xerotica obliterans is not associated with phimosis and manifests with classic clinical and pathologic features. The cause of this classic form of balanitis xerotica obliterans is unknown, but an autoimmune mechanism has been suggested.70,85–88 Patients with lichen sclerosus may have increased organ-specific antibodies (thyroid microsomal and parietal cell antibodies in women and smooth muscle and parietal cell antibodies in men).85–88 Association with autoimmune diseases, including vitiligo and alopecia areata, further supports the premise that autoimmune pathogenetic mechanisms may play an important role in this disease. Clinically, balanitis xerotica obliterans manifests as a well-defined and marginated white patch on the glans penis or prepuce that envelops or involves the urethral meatus, the navicularis, and the penile urethra, but not the bulbar urethra, resulting in urethral strictures (Fig. 15-14).86 It also may manifest as a lichenoid scale with a roughened surface. In long-standing cases, the lesion is firm because of the underlying fibrosis, which may cause phimosis in uncircumcised men.89,90 Most lesions occur on the glans penis or prepuce, but the shaft is occasionally involved. Urethral involvement may cause stricture.91 Pruritus, pain, and dyspareunia are common in vulvar lichen sclerosus et atrophicus, but balanitis xerotica obliterans is usually asymptomatic. Histologically, active lesions of balanitis xerotica obliterans show pronounced orthokeratotic hyperkeratosis accompanied by striking atrophy of the epidermis, a distinctive combination of features. Basal cell vacuolation and clefting of the dermoepidermal junction also may occur; in rare instances, bullae may be seen. Orthokeratotic plugging of cutaneous follicles, a feature of lichen sclerosus et atrophicus, is not seen in balanitis xerotica obliterans because of the absence of follicles in this area.74,75 The upper dermis is markedly edematous, and the collagen forms a homogenized band, beneath which a lymphoplasmacytic infiltrate may be present (Fig. 15-15). Fig. 15-15 Balanitis xerotica obliterans showing homogeneous collagen in upper dermis with thin band of chronic inflammation just below. The treatment of balanitis xerotica obliterans is often difficult. Circumcision, laser therapy, and topical administration of steroids, antifungal agents, and retinoids have been used with variable results. A recent report stated that after successful treatment of lichen sclerosus with long-term corticosteroids, purple to red asymptomatic angiomatoid nodules resembling the clinical features of Kaposi sarcoma developed.92 Balanitis xerotica obliterans is considered to be the most important and prevalent non–HPV-related premalignant condition and may precede, coexist with, or arise subsequent to the development of carcinoma. Whether lichen sclerosus proceeds to penile keratinizing carcinoma is a matter of debate. Lichen sclerosus is preferentially associated with non-HPV variants of squamous cell carcinoma. When lichen sclerosus is associated with malignancy, it often shows, in addition to hyperplastic epithelium, the presence of a low-grade squamous intraepithelial lesion. These findings suggest that lichen sclerosus may represent a preneoplastic condition for at least some types of penile cancer, in particular those not related to HPV.74,93,94 Whether lichen sclerosus represents a premalignant process remains a matter of some debate. Barbagli and colleagues81 retrospectively reviewed the histology of 130 patients with lichen sclerosus and reported premalignant or malignant features in 11 (8.4%). In the largest series to date, squamous cell carcinoma was found in 2.3% of 522 patients diagnosed with lichen sclerosus.89 Nasca and colleagues reported on a series of 86 patients with lichen sclerosus in which squamous cell carcinoma was subsequently found in only 5.8%.88 In all cases, epithelial dysplasia and lichen sclerosus were found adjacent to tumor foci, indicating possible histologic progression from chronic inflammation to dysplasia and eventually to malignant transformation. Although European guidelines consider lichen sclerosus to be a premalignant condition,90 no consensus has been reached on the follow-up of these patients. In 1916, Reiter described a patient who developed systemic illness with polyarthritis, conjunctivitis, and nongonococcal urethritis after an episode of bloody diarrhea. Although this was not the first reported case, the syndrome characterized by the triad of arthritis, urethritis, and conjunctivitis is now commonly referred to as Reiter syndrome. More than two thirds of patients have associated mucocutaneous lesions, supporting the argument that Reiter syndrome is better defined by a tetrad of symptoms that includes mucocutaneous lesions.95 More than 90% of patients are males, with onset of symptoms in the third and fourth decades.96 Epidemic (enteric) and endemic (urogenital) modes of presentation have been described, with the latter being much more common.96–98 Patients frequently report a history of recent sexual contact with a new partner that is followed by the development of urethritis. The less common epidemic form is secondary to enteric infection and also occurs in children. Urethritis occurs in 90% of the postdysenteric or enteric form of the disease, so it should not be assumed that urethritis and Reiter syndrome are always transmitted sexually. Chlamydia trachomatis probably is the most common cause for the sexually acquired form of Reiter syndrome, although Ureaplasma urealyticum, Shigella flexneri, Salmonella species, Campylobacter species, Yersinia enterocolitica, and Neisseria gonorrhoeae also have been implicated.95–100 Genetic susceptibility plays an important role; 60% to 80% of patients are positive for human leukocyte antigen (HLA)-B27. It is postulated that HLA-B27, either as a result of molecular mimicry or by virtue of its relation to antigens linked to genes controlling immune responses to certain infectious agents, produces an exaggerated or abnormal immune response to specific microbiologic agents that culminates in the inflammatory manifestations of the disease.101 Genital involvement occurs as part of the mucocutaneous manifestations of Reiter syndrome and is common in the sexually acquired form of the disease. The lesions take two forms: balanitis circinata and keratoderma blennorrhagica. Balanitis circinata is the more common form and occurs in up to 85% of men with the sexually acquired form of the syndrome.102–104 It consists of a painless lesion that begins as small red papules that enlarge centrifugally to form a circular or ringlike configuration. In circumcised men, the lesion is hyperkeratotic and resembles the second lesion, keratoderma blennorrhagica. Keratoderma blennorrhagica is predominantly a cutaneous lesion, most commonly affecting the palms and soles. It begins as erythematous macules that enlarge to form hyperkeratotic papules with red halos. This form is clinically and histologically similar to psoriasis, and some cases of Reiter syndrome progress to become indistinguishable from psoriatic arthritis.104 Histologically, the early lesions are indistinguishable from psoriasis vulgaris or pustular psoriasis, and they demonstrate psoriasiform hyperplasia, hyperkeratosis, parakeratosis, and neutrophilic exocytosis within the stratum corneum, with formation of spongiform pustules.105 The spongiform pustules seen in the upper epidermis are the most characteristic histologic feature of Reiter syndrome. The papillary dermis is thickened by edema and may contain a neutrophilic perivascular infiltrate. In later stages, the pustules are absent, and the epidermis shows nonspecific findings, including acanthosis, hyperkeratosis, and focal parakeratosis. Reliable distinction of Reiter syndrome from pustular psoriasis and psoriasis vulgaris may be difficult and requires clinicopathologic correlation. Various therapies used in the management of Reiter syndrome are nonsteroidal antiinflammatory drugs, antibiotics, and disease-modifying antirheumatic drugs such as sulfasalazine or methotrexate. Successful treatment of Reiter syndrome with tumor necrosis factor-α blockers has been reported.106 Peyronie disease (also called plastic induration, fibrous sclerosis, and fibrous cavernositis) manifests with painful erection accompanied by distortion, bending, or constriction of the erect penis.107–110 Observations resembling Peyronie disease were made in 1561 by the Italian anatomist Fallopius, but the first detailed description was by de la Peyronie, in a series of patients with deformities of the erect penis. Peyronie disease affects men between the ages of 20 and 80 years (median, 53 years) but is uncommon in men younger than 40 years old. The prevalence of patients presenting under the age of 40 is only 1.5%.111,112 Peyronie disease is more prevalent in patients with diabetes and urolithiasis.113 In addition, genetic predisposition, trauma of the penis, systemic vascular diseases, smoking, and alcohol consumption are other possible etiologic factors in the development of the disease.114 More than 66% of patients report painful erection. In contrast, in patients without pain, the manifesting symptom is penile bending (Fig. 15-16), which varies in duration from an overnight appearance to a few months or, in some instances, a few years. Patients concerned about the presence of tumor may seek attention after feeling a plaque. These lesions often are palpable as firm nodules or plaques on the dorsal surface of the erect penis. Examination of the flaccid penis may be unremarkable. Rarely, multiple plaques may be present. Some suggest Peyronie disease may be related to fibromatosis because of its association with Dupuytren contractures or palmar or plantar fibromatosis, seen in 10% to 20% of patients.115 Others have suggested that it may be an inflammatory fibrotic reaction secondary to urethritis. Peyronie disease also appears to be related to coital trauma and urethral instrumentation and has been associated with use of β-blockers, hypertension, diabetes, and immune reactions.116–120 It is not associated with antigens of certain HLA systems.121 Hauck and co-workers122 recently found an increased frequency of the homozygous genotype of the single nucleotide polymorphism G915C in patients with Peyronie disease in contrast to healthy controls (89.2% versus 79%). However, no significant differences in allelic frequencies of the single nucleotide polymorphism T869C were found. These results indicate that the homozygous wild type of the G915C single nucleotide polymorphism in the coding region of the TGFB1 gene, which was recently associated with elevated transforming growth factor (TGF)-β1 production and pulmonary fibrosis, may influence the predisposition to Peyronie disease. However, it does not represent a major genetic risk factor.122 The expression levels of TGF-β1 and profibrotic and antifibrotic gene products, as well as the nitric oxide–to–reactive oxygen species (NO/ROS) ratio in the tunica albuginea, appear to be essential for the formation and progression of the Peyronie disease plaque and affect the expression of multiple genes. This can be assessed with recently developed DNA-based chip arrays, and results with the Peyronie disease plaque have been encouraging. OSF-1 (osteoblast recruitment), MCP-1 (macrophage recruitment), procollagenase IV (collagenase degradation), and other profibrotic genes have been identified as possible candidate regulatory genes. Gene-based therapy for the treatment of Peyronie disease is being investigated and may eventually reduce the need for surgical intervention.123 Although some earlier studies suggested a relationship between specific HLA types and Peyronie disease, further studies failed to corroborate this association.124 In addition, Hauck and co-workers125 failed to demonstrate the occurrence in Peyronie disease of 16S rDNA, which is a highly sensitive marker for the presence of bacteria in inflammatory processes. The results of this study argue against an association between Peyronie disease and bacterial infection. Bivens and associates126 reported 6 patients with Peyronie disease and carcinoid syndrome and suggested a causal role for elevated serum serotonin levels. Guerneri and colleagues127 found chromosomal aberrations in 9 of 14 patients. Although the chief pathologic finding in Peyronie disease is fibrosis of the tunica albuginea, it does not affect the erectile tissue of the corpora cavernosa. Calcification and ossification may occur in the fibrous plaques. Histologically, Peyronie disease begins with perivascular inflammation in the loose connective tissue between the tunica albuginea and the sinusoids of the corpora cavernosa. Deposition of fibrin in the tunica albuginea may be the primary event, followed by inflammation, fibrosis, and collagenization. In surgical specimens, the histologic features are less dramatic than the clinical presentation, often consisting only of a cellular proliferation resembling fibromatosis or merely fibrosis (Fig. 15-17). Studies have shown excessive amounts of type III collagen in the plaques.128 Ultrasonography of the penis is a helpful tool to the urologist, providing anatomic detail of soft tissue structures, and recently it has been used in the assessment of Peyronie disease.129,130 Smith and colleagues130 studied ultrasonography in Peyronie disease and demonstrated tunica thickening, calcifications, septal fibrosis, and intracavernosal fibrosis in 50%, 31%, 20%, and 15% of men, respectively. Men 40 to 59 years of age were more likely to have subtunica calcifications relative to men younger than age 40. Men with septal fibrosis had fewer chronic medical conditions such as diabetes, hypertension, and coronary artery disease and presented within 1 year of disease onset. Men with septal fibrosis were less likely to have lost penile length and more likely to be able to have intercourse. Men with intracavernosal fibrosis were less likely to have penile pain but more likely to have penetration difficulty during intercourse, an additional penile deformity, or rapid onset of disease. Tunica thickening was associated with decreased ability to have intercourse.129 The clinical course is variable. The disease resolves spontaneously in fewer than one third of the patients, progresses in up to 40%, and remains stable in the rest.131 Treatment has included surgical excision of the plaques, intracavernosal plaque excision, radiotherapy, intralesional injection of interferon, steroid injections, and extracorporeal shock wave therapy.121–136 Surgical approach with tunica excision in patients with palpable lesions and penile curvature can result in impotence and decreased penile sensation. Eisenberg and associates137 and Bella and co-workers138 described a novel method of excision of such lesions with preservation of the tunica that maintained potency and penile sensation. The combination of colchicine and vitamin E (which has antifibrotic, antimitotic, and antiinflammatory effects) in modifying the early stages of Peyronie disease has been used in one study and was an effective and well-tolerated way to stabilize the disease, but more extensive studies are needed, comparing these results with other oral therapies.139 Prostheses may be required to restore potency.140 Heterotopic penile bone (os penis) is occasionally found in the plaques of Peyronie disease, particularly in elderly men.141 In children, the presence of os penis is considered a congenital anomaly related to the normal occurrence of penile bone in numerous carnivorous animals, a feature lost in humans.142 The bone is usually deposited just beneath the tunica albuginea. Cutaneous horn is a rare exophytic, conical, keratotic mass that arises in areas of chronic inflammation. Little is known about its pathogenesis, but long-standing preputial inflammation and phimosis are known to play an important role.143 It has a risk for malignant transformation into low-grade verrucous or keratinizing squamous cell carcinoma, reported in approximately 30% of patients.144 Leukoplakia of penis is rare and consists of white, verrucous plaques that can arise on mucosal surfaces. Genital lesions occur primarily on the glans or prepuce and can clinically resemble areas of lichen sclerosus. They occur more commonly in patients with diabetes, probably related to recurrent and chronic infection. Dysplastic changes have been reported in 10% to 20% of patients.145,146 Penile prostheses are surgically implanted devices that aid in erection by providing penile rigidity.147 Since their introduction in the early 1970s, the technology has greatly advanced, chiefly because of better understanding of erectile physiology and pathophysiology. These developments have resulted in widespread patient and physician acceptance of these devices, as well as a substantial reduction in complications. The indications for prosthetic implantation are organic and psychogenic impotence. Organic causes include diabetes, paraplegia, quadriplegia, and Peyronie disease. Therapeutic advances in vascular surgery and pharmacotherapy are leading to decreased use of penile prostheses in patients with organic causes because other modalities offer better results.147 The two general categories of penile prosthesis are malleable devices and inflatable devices. These differ from one another in their construction and operation.147–149 Malleable devices provide simplicity of implantation and have no mechanical parts that may fail. They require very little manual dexterity because they merely need to be bent upward before use. They are disadvantageous because neither the size nor the rigidity of the penis changes. Inflatable devices are based on hydraulic principles that allow inflation for sexual intercourse with deflation in the detumescent phase. These devices are more difficult to implant and have limited life span because of eventual mechanical failure. Complications of penile prostheses may occur during surgery (usually crural or corporal perforation), postoperatively (mainly infection or component failure), or later owing to device erosion. Although more than 90% of patients report satisfaction, the reoperative rate is as high as 44%. Slightly lower rates have been reported recently and are expected to improve further with new designs and surgical advances.150–152 Besides penile erection, endourethral prostheses for urethral stricture have been used in clinical practice with variable success.153 Priapism is defined as prolonged painful erection unrelated to sexual desire. Typically, pain and tenderness result after 6 to 8 hours and are related to ensuing ischemia. Priapism is a relatively uncommon condition that may manifest as a medical emergency associated with significant pain and anxiety in the venoocclusive or low-flow variant.154 Priapism may be primary, secondary, or idiopathic. Secondary causes include genital trauma, thromboembolism, hemostasis, and leukostasis (fat embolism, sickle cell anemia, leukemia), neurologic defects (anesthetic agents, spinal cord injury, and autonomic dysfunction), infiltration by cancer, pharmacologic effects (alcohol, drugs acting on central nervous system, total parenteral nutrition), and intracavernous injections for diagnostic procedures (papaverine hydrochloride, prostaglandin E, and phentolamine).154,155 Pohl and associates156 reviewed 230 cases from the literature; more than 33% were idiopathic, 21% were reactions to drugs and alcoholism, 12% were caused by trauma, 11% were caused by sickle cell anemia (an important cause of priapism in children), and less than 1% were due to neoplasms. Priapism can be caused by hematologic malignancy with hypercoagulation or metastatic disease involving the corpora cavernosa with thrombosis of the venous outflow from the penis.157 Data on pathologic findings in priapism are extremely limited. The corporeal tissue may be edematous, indurated, and ultimately sclerotic. Ultrastructural examination reveals interstitial edema within 12 hours, destruction of sinusoidal endothelium and exposure of basement membrane with adherence of platelets by the end of 24 hours, and, finally, vascular thrombi associated with ischemic necrosis of smooth muscle tissue at 48 hours.158 Besides control of the precipitating factors, treatment includes conservative therapy with analgesics, sedatives, and fluids; control of pain with penile block; aspiration and injection of anesthetic agent, injection of α-adrenergic receptor agonists, intracavernosal injection of thrombolytic medications, and surgery for cavernosal shunt.159,160 Gonorrhea is caused by N. gonorrhoeae, a gram-negative, nonmotile, non–spore-forming, biscuit-shaped diplococcus. The term gonorrhea was coined by Galen in the second century and means “flow of semen,” referring to the exudate of gonorrheal urethritis. The disease was recorded before the common era in descriptions by Hippocrates and Celsius. The latter treated gonorrheal strictures by catheterization.161 In men, gonorrhea typically produces urethritis with urethral discharge, which may be profuse, purulent, or scant (gleets), and burning micturition.161,162 This disease is sexually acquired, and the risk for infection increases as the number of sexual partners increases. The penis is involved only as a complication of the disease, with cutaneous lesions, infection of the median raphe, penile abscess, and gonococcal tysonitis (inflammation of the preputial glands).163,164 The chief complication is urethral stricture. Laboratory tests are essential because the disease is frequently mimicked by, and coexists with, chlamydial infection.165 Standard diagnostic procedures include Gram stain, culture, and microbial susceptibility testing.161,162 The Centers for Disease Control and Prevention (CDC) has given guidelines for its treatment, consisting primarily of antibiotic therapy.166 Treatments for uncomplicated urogenital, anorectal, or pharyngeal gonococcal infections include cephalosporins and fluoroquinolones. Fluoroquinolones should not be used in patients who live in or may have contracted gonorrhea in Asia, the Pacific islands, or California or in men who have sex with men. Gonorrhea infection should prompt physicians to test for other sexually transmitted diseases, including HIV.167,168 Syphilis is one of the most fascinating diseases affecting humans and has been investigated and described by clinical scholars, playwrights, and poets, including Fracastoro, who in 1530 wrote in his poem about the suffering shepherd Syphilis.169 Although the disease was thought to be declining in incidence, it seems to have made a comeback in recent years.170–173 Syphilis is produced by Treponema pallidum, a microaerophilic gram-negative spirochete, after a 9- to 90-day incubation period. In its classic form, untreated syphilis occurs in three stages: primary, secondary, and tertiary. In the primary stage, penile involvement commences as a tiny papule, usually at the site of genital trauma on the glans penis, coronal sulcus, prepuce, frenulum, or shaft. In homosexual men, the lesion may occur in the anal canal or rectum. The lesion progresses through the papular phase into an ulcerated chancre. The classic chancre is a single round, painless ulcer with sharp margins and a clean, indurated base (Fig. 15-18). Lymphadenopathy develops within a week, and the nodes are typically painless, rubbery, and nonsuppurative. With or without therapy, the primary ulcer heals within 6 to 8 weeks.169,170 Dark-field microscopy is the mainstay of diagnosis during this phase of the disease because the antibody response lags.170 Biopsy is usually not necessary but may be performed if the diagnosis of syphilis is not suspected. Histopathologic features include epidermal ulceration with acanthosis at the margins. The submucosa or dermis contains an inflammatory infiltrate of lymphocytes and plasma cells that is mostly diffuse but may be concentrated around blood vessels and associated with pronounced proliferation of endothelial cells (Fig. 15-19).169,174 The Warthin-Starry or Levaditi stain reveals spirochetes in the epidermis or in the dermis around capillaries. The organisms typically have 8 to 12 convolutions, but reticulin fibers may mimic them and interpretation must be cautious.174 The lymph nodes exhibit follicular hyperplasia, with many plasma cells and endothelial proliferation. Special stains may show numerous spirochetes in lymph nodes. Fig. 15-19 Syphilis. Dense chronic inflammatory infiltrate with predominantly plasma cells and endothelial cell hyperplasia. In secondary syphilis, penile involvement is usually part of the systemic mucocutaneous manifestations of this stage. The T. pallidum organisms circulate in the blood and lymphatic systems for 6 weeks to 6 months after the primary stage, producing symmetric skin lesions and generalized lymphadenopathy. The skin lesions are maculopapular, annular, and usually hyperpigmented. Secondary syphilis may manifest with nodular lesions.175,176 Condyloma latum and mucous patches are included in the constellation of mucocutaneous lesions. Smears from these lesions should be examined by dark-field microscopy for organisms. A biopsy may yield variable histologic features and by itself is nonspecific, because in up to 25% of patients the plasma cell infiltrate and capillary endothelial proliferation typical of syphilis are absent.177 The lesions lacking plasma cells and endothelial proliferation may mimic other cutaneous diseases such as lichen planus or psoriasis. A pronounced lymphocytic response may also be mistaken for mycosis fungoides.178 The epidermal changes include parakeratotic scales, acanthosis, ulceration, spongiosis, exocytosis, dyskeratosis, and basal vacuolation. Condyloma latum shows prominent epithelial hyperplasia that may become pseudoepitheliomatous with ulceration and exocytosis with neutrophils.179 Until recently, syphilis was considered a major cause of penile cancer, but the possible role of syphilis was discarded without much debate with the acceptance of certain HPVs primarily involved as etiologic agents. A recent study with logistic regression showed that patients with penile cancer did not have a syphilis history significantly more often than control colon and stomach cancer patients.180 Herpes (from Greek, meaning “to creep”) simplex virus infection involving the genital system is an important sexually transmitted disease that may have a causal role in cervical carcinoma and has high morbidity and mortality in infants. The virus is a double-stranded DNA virus that has two subtypes. Herpes simplex virus type 1 produces oral lesions, whereas herpes simplex virus type 2 predominantly affects the genitalia; only 10% to 25% of herpes simplex virus type 2 infections produce oral lesions.181,182 Herpes genitalis and infections caused by HPV are increasingly common, particularly in young, sexually active people. However, herpes simplex virus infection remains the most common infectious cause of genital ulceration, with evidence that many infections are asymptomatic.182,183 Clinical manifestations are more severe and fulminant in the first episode than in recurrent disease.184,185 Initial episodes often have systemic symptoms (fever, malaise, and headache) and affect multiple extragenital and genital sites. Pain, itching, urethral discharge, dysuria, and tender lymphadenopathy are the most common local symptoms. The genital lesions appear as multiple vesicles with erythematous bases that may coalesce, rupture, form pustules, and eventually become encrusted (Fig. 15-20, A). Asymptomatic viral shedding may occur for a prolonged period.186 The clinical diagnosis may be confirmed by scraping the lesion and obtaining a Tzank smear (stained with Wright-Giemsa, toluidine blue, or Papanicolaou procedures), which reveals large multinucleated giant cells with ballooning degeneration and characteristic intranuclear inclusions. However, this method cannot differentiate between herpes simplex virus and varicella-zoster infection. For this distinction, other techniques such as immunofluorescent antigen detection, virus isolation by culture, and serology are necessary.187 Fig. 15-20 A, Herpes simplex. B, Herpes simplex vesicle with multinucleation, nuclear molding, and margination. Histologically, herpes simplex and varicella-zoster lesions are identical, characterized by unilocular or multilocular intraepidermal vesicles produced by profound acantholysis (see Fig. 15-20, B).188 The vesicles contain proteinaceous material and are surrounded by epidermal cells with reticular and ballooning degeneration, features that are hallmarks of herpes infection but absent in other vesiculobullous diseases that may enter into the differential diagnosis. Cytopathic changes may be evident in adnexal and pilosebaceous structures, endothelial cells, and fibroblasts, but are typically seen in the epidermal cells, which exhibit chromatin margination and inclusion bodies (ranging from small demarcated acidophilic bodies to large, homogeneous, ground-glass acidophilic to basophilic bodies) surrounded by halos. Atypical clinical manifestations of herpes simplex virus may occur in HIV-positive immunocompromised patients presenting with tumor-like nodules or condylomatous or hypertrophic lesions, rather than classic ulcer. Such unusual presentations raise the risk for misdiagnosis and a delay in appropriate treatment. Therefore it is important to be aware of these unusual presentations to provide a correct diagnosis and prompt, effective treatment for herpes simplex virus. Several studies suggest that aggressive treatment of herpes simplex virus in combination with highly active antiretroviral therapy (HAART) provides a significant survival benefit. Pathobiology mechanisms of unusual and exaggerated tumor-like inflammatory response are not completely elucidated.189 Complications of herpes include neonatal complications; nervous system abnormalities (aseptic meningitis, encephalitis, radiculopathies)190; extragenital lesions on buttocks, groin, thighs, or other sites; disseminated infection resulting in arthritis, hepatitis, or hematologic disorders; superinfections; intractable nonhealing ulcers in patients with acquired immunodeficiency syndrome (AIDS); and possibly cervical cancer in women. Treatment options are limited. Acyclovir and phosphonoformate trisodium are effective in decreasing the intensity and duration of both primary and recurrent episodes but are not curative. Herpes zoster is a common dermatologic infection and predominantly affects the trunk in up to 50% to 60% of patients, followed by the head region (10% to 20%), with sacral dermatomes involved in only up to 5% of patients. Penile zoster is neither commonly seen by dermatologists nor reported in dermatologic journals.191 The diagnosis of herpes zoster is made clinically; however, laboratory confirmation is necessary only in atypical inconclusive clinical cases. Patients with penile herpes zoster present with vesicular rash involving S2 to S4 dermatomes. Postherpetic neuralgia is the most frequently reported complication, and risk factors include older age, more severe acute pain, and greater rash severity. Bladder dysfunction and urinary retention have been reported in patients with herpes zoster, and penile herpes zoster should not be overlooked in patients with unilateral vesicular rash. Lymphogranuloma venereum is a sexually transmitted disease caused by C. trachomatis subtypes L1, L2, and L3. It was established as a clinicopathologic entity separate from other venereal diseases in 1913 by Durand, Nicolas, and Favre.192 Lymphogranuloma venereum is sporadic in the United States and most European countries, but it is highly prevalent in Africa, Asia, and South America. Rarely, it is associated with HIV infection.193 Like syphilis, lymphogranuloma venereum has three stages: primary genital stage; secondary inguinal stage characterized by acute lymphadenitis with bubo formation; and a rare chronic tertiary stage with genital ulcers, fistulas, elephantiasis, and rectal strictures.194,195 After an incubation period of 3 days to 6 weeks, the lesion begins as a papule, which transforms into a pustule and heals without treatment; therefore in 50% of patients, the penile lesion is not clinically examined. The pathognomonic clinical sign is inguinal lymphadenopathy, commonly known as bubo. It is painful, usually unilateral (66%), and may enlarge to form an abscess and rupture (33%).194–196 The histologic features are nonspecific and nondiagnostic. The ulcer is coated with exudate and neutrophils; the base is composed of granulation tissue, with a mixed inflammatory infiltrate containing large mononuclear cells with occasional granulomas.197 Giemsa stains may demonstrate purple chlamydial inclusions in the macrophages, but this procedure lacks specificity and sensitivity. The lymph nodes show follicular hyperplasia and elongate stellate abscesses similar to those of cat-scratch disease, tularemia, and fungal and atypical mycobacterial infections. Culture of the organisms by aspirating the lymph nodes is a useful method of detection, but is technically difficult and costly. The Frei test is no longer used, but serologic tests have gained wide acceptance for diagnosis of lymphogranuloma venereum.198 Genetic testing is now widely available and is rapid and specific. Lymphogranuloma venereum can be confirmed by the detection of lymphogranuloma venereum–specific DNA from specimens such as bubo aspirates, ulcer swabs, urethral swabs, first voided urine, and rectal biopsy material.199
Penis and scrotum
Penis
Normal anatomy and histology
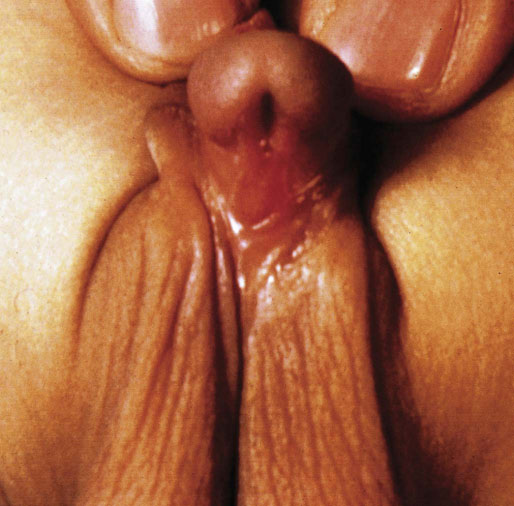
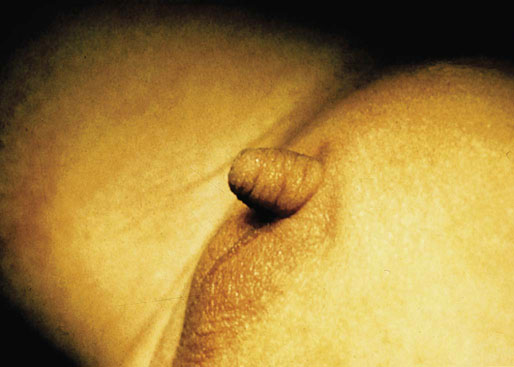
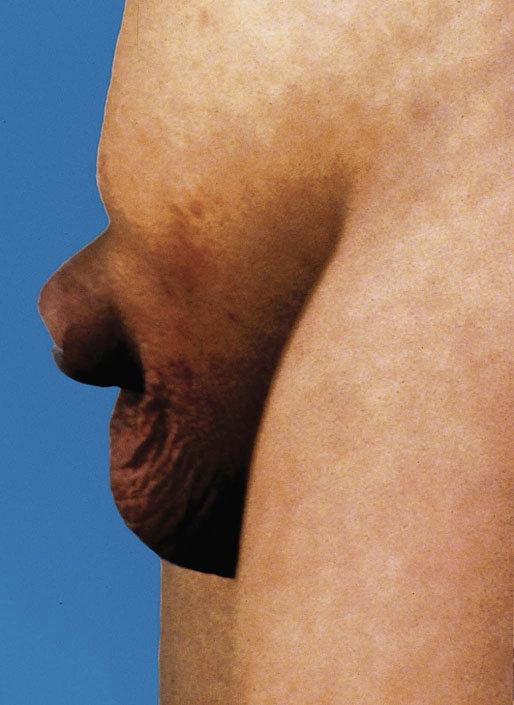
Congenital anomalies
Nonneoplastic diseases
Inflammation
Phimosis and paraphimosis
Fibroepithelial (lymphedematous) polyp
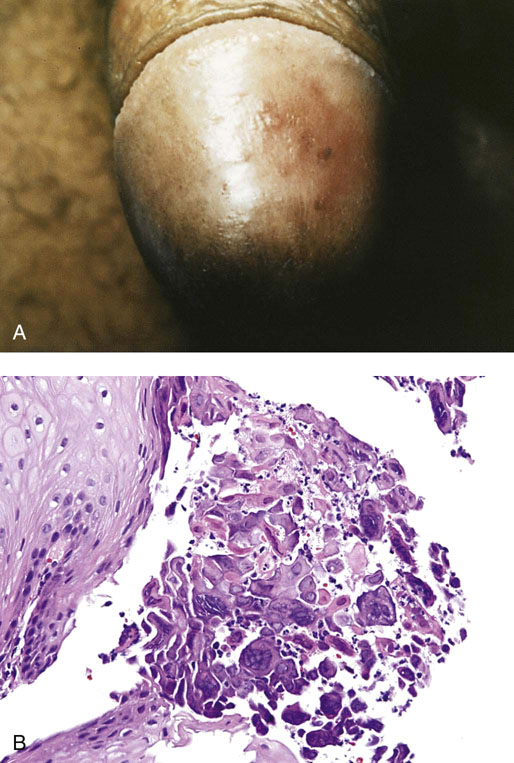
Plasma cell balanitis (Zoon balanitis)
Balanitis xerotica obliterans (penile lichen sclerosus)
Reiter syndrome
Peyronie disease
Cutaneous horn and leukoplakia
Penile prosthesis
Priapism
Infections
Gonorrhea
Syphilis
Herpes simplex and zoster
Lymphogranuloma venereum
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree