Douglas A. Husmann, MD
General Comments On Pediatric Renal Trauma
The Pediatric Kidney: Traumatic Renal Injuries and Congenital Renal Anomalies
The pediatric kidney is believed to be more susceptible to trauma, due to a decrease in the physical renal protective mechanisms found in childhood. Specifically, in contrast with the adult, the pediatric kidney is protected by an immature, more pliable thoracic cage, weaker abdominal musculature, less perirenal fat, and it sits in a lower abdominal position. Whether the incidence of renal injury following blunt abdominal trauma is truly increased in the pediatric versus the adult patient population is controversial, with statistical evaluations revealing mixed results, and the question remains in play (Brown et al, 1998; Chopra et al, 2002; McAleer et al, 2002a; Heyns, 2004).
What is known however is that preexisting renal abnormalities (i.e., ureteropelvic junction [UPJ] obstruction, hydroureteronephrosis, horseshoe kidney) are three- to fivefold more common in pediatric patients undergoing a screening CT scan for trauma than in the adult population (Brown et al, 1998; Chopra et al, 2002; McAleer et al, 2002a; Heyns, 2004).
Classically, patients with a preexisting congenital renal abnormality present with a history of hematuria disproportionate to the severity of trauma. Although it has been hypothesized that a preexisting congenital genitourinary anomaly would be associated with a higher stage of renal injury, this has not been documented to be true, with the majority of the patients still sustaining only renal contusions or minor renal fracture (Al-Qudah and Santucci, 2006; Chopra et al, 2002; McAleer et al, 2002a).
Trauma-Induced Hematuria: Pediatric versus Adult Patients
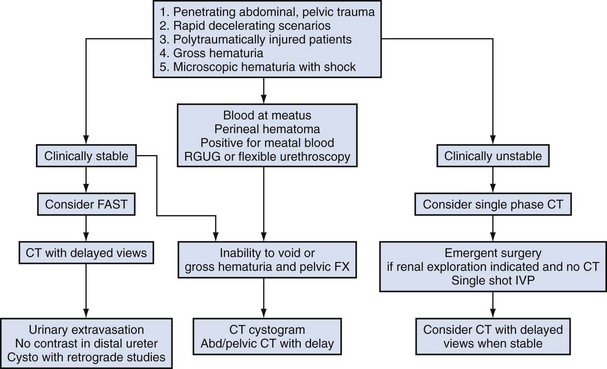
Two major clinical findings suggest the need to work up the genitourinary tract of an adult for a possible injury following trauma: Is there gross hematuria or is there microscopic hematuria (>50 red blood cells [RBC]/high-power field [HPF]) with shock (systolic pressure <90 mm Hg)? In adults, if physicians use only these two questions to decide who to screen for a traumatic genitourinary injury, they will find 98% of the clinically significant genitourinary (GU) injuries following blunt trauma and 90% of GU injuries associated with penetrating trauma (Mee et al, 1989; Heyns, 2004; Santucci et al, 2004a). Indeed, the yield in the adult population with just those two questions is significant, with 30% of the patients presenting with gross hematuria and 10% of the patients with microscopic hematuria associated with shock found to have a radiologically definable GU injury (Mee et al, 1989; Heyns, 2004; Santucci et al, 2004a). In contrast, in children there is poor correlation relating the presence of hematuria to the presence of renal injuries. Indeed, some studies have found that two thirds of children sustaining a grade 2 or higher renal injury will have completely normal urinalysis (Morey et al, 1996; Buckley and McAninch, 2004, 2006; Santucci et al, 2004a). The correlation of hypotension to the extent of injury is also problematic, with the sympathetic tone in children being able to sustain a normal blood pressure despite significant blood loss. In fact, in children serial hemoglobin/hematocrit (Hb/Hct) values will demonstrate significant decreases before orthostatic hypotension develops (Quinlan and Gearhart, 1990). Notably, 90% of the pediatric patients with a clinically significant traumatic renal injury will have coexisting injuries to either the thoracic contents, intra-abdominal organs, or orthopedic (ribs, spine, pelvic, or femur) fractures (Levy et al, 1993; Buckley and McAninch, 2004; Heyns, 2004; Sahin et al, 2004; Santucci et al, 2004b). In essence, in the pediatric patient presenting with a history of trauma, the clinical history combined with the presence of associated injuries becomes paramount in determining the need to assess for possible GU injuries (Morey et al, 1996; Buckley and McAninch, 2004, 2006; Santucci et al, 2004a).
Indications for Imaging the Genitourinary Tract Following Trauma in the Pediatric Patient
Santucci and associates reviewed all of the published data regarding traumatic renal injuries in children. The outcome of this excellent review article found that the screening criteria outlined in Table 138–1 were highly reliable and cost effective (Mee and McAninch, 1989; Mee et al, 1989; Herschorn et al, 1991; Heyns, 2004; Santucci et al, 2004a, 2004b; Wu and Gaines, 2007).
Table 138–1 Indications for Radiographic Assessment of Pediatric Patient for Possible Genitourinary Injury
HPF, high-power field; RBC, red blood cells.
Radiographic And Endoscopic Assessment And Treatment Of Upper Tract Genitourinary Injuries
FAST: Focused Assessment with Sonography for Trauma
Because of its low cost, wide availability, and freedom from ionizing radiation, the use of ultrasonography (US) has become a major screening tool in pediatric level 1 trauma centers. Use of the FAST exam to detect renal injuries has a reported specificity range of 95% to 100%, (the ability to diagnose true negatives—kidneys without a clinically significant traumatic injury). However, the sensitivity (the ability to diagnose true positives—kidneys with a clinically significant traumatic injury) is highly variable and extremely operator dependent, with published sensitivity results ranging widely from 22% to 85% (McGahan et al, 1999; Jang et al, 2004; Sirlin et al, 2004; Suthers et al, 2004; Nural et al, 2005; Lee et al, 2007b; Bent et al, 2008). The clinical usefulness of a FAST scan is best when combined with serial physical examinations. If both the FAST exam and serial physical examinations are within normal limits for 24 hours, the combination of these findings will virtually rule out the presence of significant renal and/or intra-abdominal injuries (McGahan et al, 1999; Jang et al, 2004; Sirlin et al, 2004; Suthers et al, 2004; Nural et al, 2005; Lee et al, 2007b; Bent et al, 2008).
Abdominal and Pelvic Computed Tomography and Single-Shot Intravenous Pyelography (IVP)
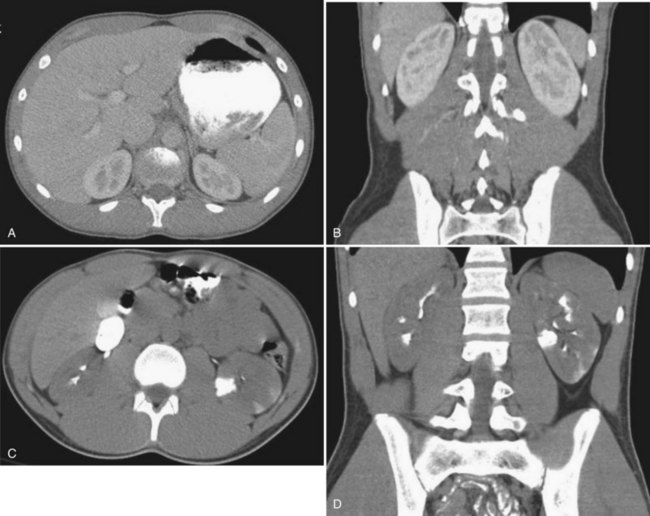
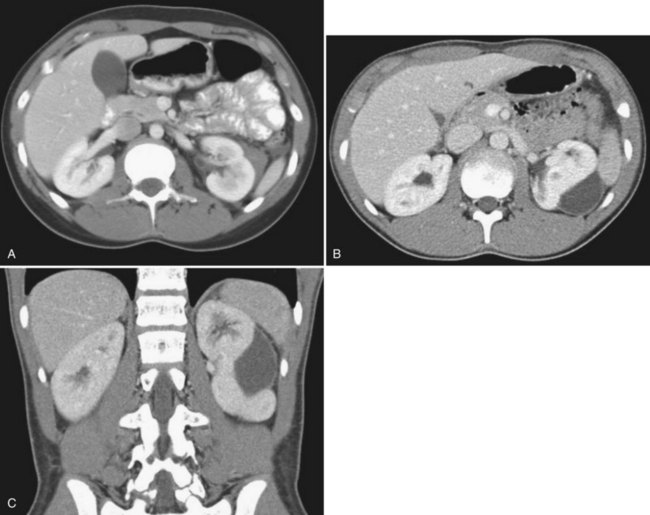
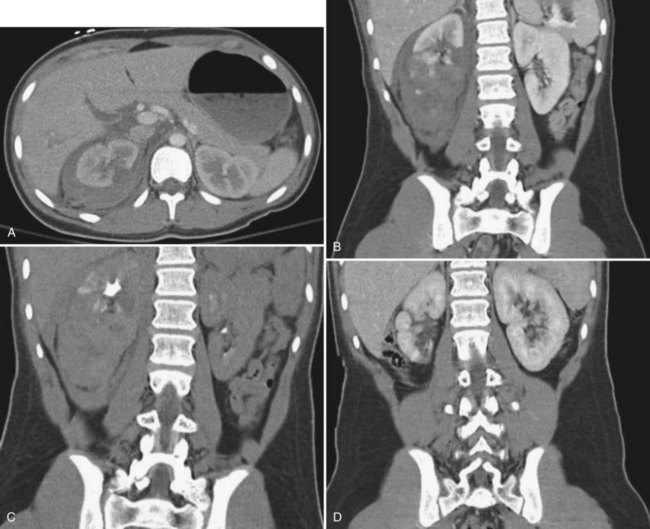
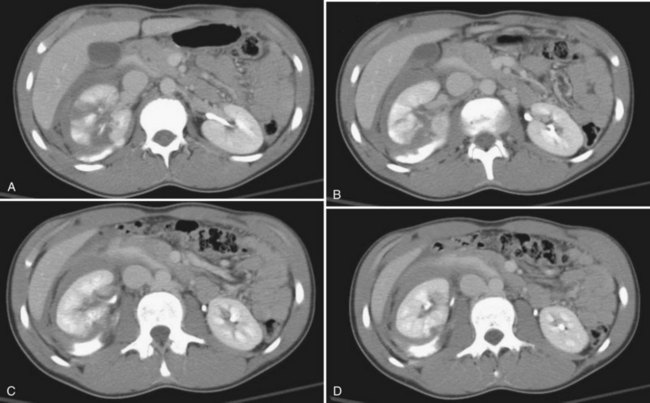
The patient’s hemodynamic stability determines whether, when, and occasionally what type of imaging studies can be done. The most sensitive and specific radiologic test to rule out a genitourinary injury is a triphasic abdominal and pelvic computed tomography (CT) scan (precontrast study, followed by a CT study immediately after injection of contrast [1.5 to 2 mL/kg], and then a 10-minute delayed study). See Table 138–2 for the classification of renal trauma and Figures 138-1 through 138-5 for examples (Mee et al, 1989; Stein et al, 1994; Morey et al, 1996; Brown et al, 2001; Buckley and McAninch, 2004, 2006; Heyns, 2004; Santucci et al, 2004b; Al-Qudah and Santucci, 2006; Lee et al, 2007b). In the clinically labile patient, a single-phase CT scan with the study taken immediately following injection of contrast is the mainstay. This test is beneficial in determining renal perfusion and the presence of major renal fractures; it will, however, be unable to accurately determine the presence of urinary extravasation and, in our experience, will miss the majority of the isolated ureteral injuries (Boone et al, 1993). In the unstable patient requiring emergent laparotomy, once the patient is stabilized in the operating room (OR), a one-shot IVP (2 mL/kg intravenous bolus of contrast) with the x-ray taken 10 to 15 minutes following injection may be beneficial if retroperitoneal exploration is considered. The authors would caution the reader regarding the quality of the single-shot IVP; this study is frequently a suboptimal examination with poor excretion/visualization of contrast, due to the patient’s clinical status. The chief benefit of the single-shot IVP may be to detect a normally functioning contralateral kidney if unilateral nephrectomy is a consideration. As an alternative to the single-shot IVP, the patient can be stabilized and a delayed CT evaluation obtained. Definitive surgical repair of any GU injury is subsequently deferred for 12 to 24 hours (Azimuddin et al, 1997; Heyns, 2004).
Table 138–2 Grading of Renal Injuries
| GRADE OF RENAL INJURY | DESCRIPTION |
|---|---|
| 1 | Renal contusion or subcapsular hematoma |
| 2 | Nonexpanding perirenal hematoma, less than 1-cm parenchymal laceration, no urinary extravasation, all renal fragments viable |
| 3 | Nonexpanding perirenal hematoma, greater than 1-cm parenchymal laceration, no urinary extravasation, renal fragments may be viable or devitalized |
| 4 | Laceration extending into the collecting system with urinary extravasation, renal fragments maybe viable or devitalized or Injury to the main renal vasculature with contained hemorrhage |
| 5 | Completely shattered kidney; by definition, multiple major lacerations of greater than 1 cm associated with multiple devitalized fragments or Injury to the main renal vasculature with uncontrolled hemorrhage; renal hilar avulsion |
Indications and Use of Arteriography: Management of Persistent/Delayed Hemorrhage and Persistent Urinoma
Approximately 25% of patients with a grade 3 to 4 renal trauma, managed in a nonoperative fashion, will develop persistent or secondary (delayed) hemorrhage (Wessells et al, 1997b; Dinkel et al, 2002; Goffette and Laterre, 2002; Kansas et al, 2004; Sofocleous et al, 2005, Al-Qudah and Santucci, 2006). Classically, delayed hemorrhage develops 5 to 14 days postinjury but may occur up to 1 month following the insult. Delayed hemorrhage usually arises from the development of arteriovenous fistulas or pseudoaneurysm malformations. In this scenario, the initial bleeding is tamponaded by the surrounding hematoma; as the hematoma resolves and liquefies recurrent bleeding develops. Unlike the arteriovenous fistulas noted after renal biopsy, where a spontaneous resolution rate of greater than 70% is found, the vast majority of arteriovenous fistulas occurring after renal trauma will not spontaneously resolve, with almost all cases of delayed hemorrhage secondary to trauma requiring active intervention (Heyns and Van Vollenhoven, 1992; Dinkel et al, 2002; Goffette and Laterre, 2002; Heyns, 2004; Sofocleous et al, 2005; Al-Qudah and Santucci, 2006; Breyer et al, 2008). Superselective angiographic embolization of isolated renal artery branches for persistent or secondary hemorrhage has a success rate approaching 80%. The percentage of salvaged renal function postembolization varies depending upon the degree of the initial injury, but the median reported value is 30%. Surgery required following angiography is due to one of three problems: persistent or repetitive bleeding, postembolization abscess, or persistent urinary fistula from an isolated renal segment. Angiographic embolization is currently the preferred treatment method for persistent or delay bleeding with nonoperative management protocols. Surgical exploration is reserved for embolization failure (Heyns and Van Vollenhoven, 1992; Dinkel et al, 2002; Goffette and Laterre, 2002; Heyns, 2004; Sofocleous et al, 2005; Al-Qudah and Santucci, 2006; Breyer et al, 2008).
Angiographic embolization will, on occasion, also be used to treat persistent urinary fistulas. In this scenario, a functional transected fragment of a grade 4 or 5 renal injury is completely separated from the renal collecting system, and a persistent urinary fistula/urinoma has developed despite management by double-J stent and percutaneous nephrostomy tube placement. In these rare patients, selective angiographic embolization will resolve the urinary fistula by necrosing the isolated functional renal fragment (Pinto and Chimeno, 1998; Heyns, 2004).
Postembolization syndrome is a well recognized and self-limiting condition manifested by pyrexia (up to 40° C), flank pain, and an adynamic ileus. Symptoms will usually resolve within 96 hours after the embolization. Unlike angioinfarction for renal tumors, where up to 60% of patients may develop postembolization syndrome, approximately 10% of patients following angioinfarction after a traumatic injury develop this complication. The decrease in the frequency of this syndrome following trauma is believed to be secondary to fewer pyrogens being released from the already partially necrotic tissue (Oesterling et al, 1986; Kehagias et al, 1998; Kalman and Varenhorst, 1999; Heyns, 2004; Mitra et al, 2004; Sofocleous et al, 2005; Breyer et al, 2008). The problem faced with persistent pyrexia following embolization is the need to rule out bacterial seeding of the necrotic tissue. It is therefore mandatory in the presence of a febrile response following embolization to obtain blood and urine cultures. Consideration for a repeat CT scan with possible aspiration, culture, and drainage of the hematoma/urinoma should be given if symptoms persist for greater than 96 hours (Sofocleous et al, 2005; Breyer et al, 2008).
Indications and Use of Retrograde Pyelography, Percutaneous Nephrostomy, and Perinephric Drain Placement: Diagnosis of Ureteropelvic Junction Disruptions, Renal Pelvis Lacerations, and Management of Symptomatic Urinomas and Perinephric Abscesses
Two indications exist for the use of retrograde pyelography following renal trauma: (1) the need to diagnose a partial/total ureteral disruption or renal pelvic laceration and (2) the need to aid in the management of a symptomatic urinoma (Boone et al, 1993; Kawashima et al, 1997; Heyns, 2004; Santucci et al, 2004b). Concern for a ureteral injury usually arises when a CT scan reveals urinary extravasation and no ipsilateral distal ureter is seen. In the presence of these CT findings, a retrograde pyelogram is mandatory. This study is performed to either confirm or refute the diagnosis of a ureteropelvic junction disruption or laceration of the renal pelvis, both of which require operative exploration and repair. If no evidence of these injuries is present we recommend a ureteral stent be placed following the retrograde pyelogram. This recommendation is based on prior studies revealing that patients with trauma-induced urinary extravasation who have absence of contrast in the ipsilateral distal ureter are at a higher risk for developing a symptomatic urinoma if left unstented (Boone et al, 1993; Kawashima et al, 1997; Chopra et al, 2002; McAleer et al, 2002b; Smith et al, 2003; Santucci et al, 2004b; Al-Qudah and Santucci, 2006; Broghammer et al, 2006; Cannon et al, 2008).
The second consideration for intervention with cystoscopy and retrograde pyelography is the presence of a symptomatic urinoma. Although most post-traumatic urinomas are asymptomatic and have a spontaneous resolution rate approaching 85%, urinomas will occasionally persist. Symptomatic urinomas will develop a classic triad of findings: ipsilateral flank pain, adynamic ileus, and a low-grade temperature. Management of these patients is by endoscopic intervention, with cystoscopy, retrograde pyelography, placement of a ureteral stent, urethral catheter drainage, and intravenous antibiotics. When a ureteral stent is placed in conjunction with temporary placement of a urethral catheter, greater than 90% of the symptomatic urinomas will resolve (Al-Ali and Al-Hajaj, 2001; Alskikafi et al, 2006). Classically, the urethral catheter is removed 3 to 5 days after the patient’s clinical symptoms have abated. Intravenous antibiotics are discontinued and prophylactic antibiotics initiated at the time of urethral catheter removal. The author removes the ureteral stent 4 to 6 weeks postinjury and will maintain oral prophylactic antibiotic coverage for 48 hours after stent removal. It should be noted that both percutaneous nephrostomy drainage and internal stenting are equally efficacious for the treatment of symptomatic urinomas (Husmann and Morris, 1991; Husmann et al, 1993b; Philpott et al, 2003; Bozeman et al, 2004; Heyns, 2004; Keller et al, 2004; Al-Qudah and Santucci, 2006). The advantage of an internal stent is that it prevents possible dislodgment of the drainage tube and the need for external drainage devices. The two major disadvantages of internal drainage are that both stent placement and removal, in the pediatric patient population, require general anesthesia. In addition, the small-size ureteral stents (4 to 5 Fr) placed in young children may become blocked with blood clots from the dissolving hematoma, resulting in persistence of the urinoma (Husmann and Morris, 1991; Husmann et al, 1993b).
The development of a perinephric abscess postrenal injury is extremely rare, occurring in less than 1% of injuries following blunt renal injuries and in 5% of renal injuries caused by penetrating renal trauma. It is more commonly seen in patients when there is a confluence of injuries; specifically, a devitalizing grade 3 to 5 renal injury, in conjunction with either duodenal, pancreatic, or colonic injuries or, occasionally, in patients with large areas of traumatic soft tissue loss requiring frequent debridement. On rare occasions, a perinephric abscess may arise from seeding of a perinephric hematoma by an infected venous line or by ascending bacteria following bladder colonization (Husmann and Morris, 1991; Husmann et al, 1993b).
Symptoms of a perinephric abscess are intermittent febrile spikes, flank pain, persistent ileus, and elevated white count. These symptoms may exactly mimic those associated with an undrained symptomatic urinoma. In the absence of gas within the soft tissues, the differential diagnosis between a symptomatic urinoma and perinephric abscess may be very difficult. The author chooses to treat all patients with these findings for a symptomatic urinoma and will resort to additional percutaneous drainage of the perinephric fluid collection only if there is gas in the soft tissue at the time of initial diagnosis, or if the patient’s febrile course persists after 72 hours of drainage through a ureteral stent and urethral catheter or nephrostomy tube (Husmann and Morris, 1991; Husmann et al, 1993b; Al-Qudah and Santucci, 2006).
Follow-up Radiographic Imaging after Renal Trauma: The ALARA Concept: As Low as Reasonably Achievable
Recommendations for follow-up renal imaging in the pediatric patient sustaining renal trauma has significantly changed over the past 5 years. This alteration is due to concerns regarding the future risk of radiation-induced malignancy (Brenner and Hall, 2007; Malcom et al, 2008; Shah and Platt, 2008; Eeg et al, 2009). In the recent past, a repeat CT scan for all grade 3, 4, and 5 renal injuries was recommended during the acute healing phase, usually 3 to 5 days postinjury and again at 3 months. The routine use of a CT scan 3 to 5 days post-trauma has been abandoned in an effort to decrease radiation exposure to children (Alskikafi et al, 2006; Malcom et al, 2008; Eeg et al, 2009). The principle that radiation exposure for diagnostic and follow-up purposes should be kept to a minimum is known as ALARA—as low as reasonably achievable. This concept currently permeates all of pediatric practice and has greatly impacted follow-up recommendations for post-traumatic injury. Presently, a CT scan is repeated in the acute post-traumatic phase only for patients who have symptoms; specifically, persistent or new onset of fever, persistent ileus, worsening flank pain, or persistent gross hematuria greater than 72 hours after the traumatic insult (Santucci et al, 2004b; Al-Qudah and Santucci, 2006; Buckley and McAninch, 2006; Bent et al, 2008; Malcom et al, 2008). Although some authors have advocated performing a screening ultrasonogram in this latter situation, with a CT scan used in the presence of abnormalities, the author and colleagues have frequently found the US study, in this situation, to be inconclusive, and it adds to the financial cost of patient evaluation and delays the inevitable CT scan. Based on our personal experience, we would preferentially recommend a CT scan for reevaluation if the clinical circumstances suggest reevaluation is necessary (Bent et al, 2008; Malcom et al, 2008; Eeg et al, 2009).
Currently no radiographic follow-up is recommended for grade 1 to 2 renal injuries, and renal ultrasonography at 3 months is recommended for grade 3 lacerations where all fragments are viable. There is controversy regarding radiologic follow-up for grade 3 renal lacerations associated with devitalized fragments, grade 4, and salvaged grade 5 renal injuries (Bent et al, 2008; Dunfee et al, 2008; Malcom et al, 2008; Eeg et al, 2009). Although most authors recommend performing a repeat CT/magnetic resonance imaging (MRI) scan at 3 months, others advocate US, CT, or MRI be used only in the presence of abnormalities (Eeg et al, 2009). Our personal experience is that a renal US will inevitably be abnormal following high-grade renal injuries and will prompt additional evaluations. For cost-beneficial purposes, we will preferentially perform a CT evaluation, although consideration for a MRI study can be given in the older child when sedation/anesthesia would not be necessary. The delayed study, 3 months postinjury, is done to document resolution of the urinary extravasation, assess the anatomy of the healed kidney, and rule out any occult complications (El-Sherbiny et al, 2004; Bent et al, 2008; Dunfee et al, 2008; Malcom et al, 2008; Eeg et al, 2009).
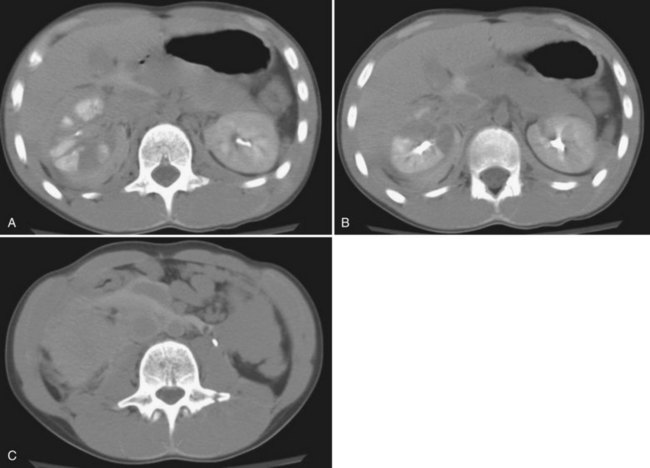
Serial dimercaptosuccinic acid (DMSA) scans obtained post-trauma have revealed that little, if any, renal parenchyma recovers function 1 week following injury; therefore nuclear renography obtained any time 1 week post-trauma results in a valid prognosis of renal function and can aid in the diagnosis of severe renal contusion versus a nonfunctional renal fragment following grade 3 and 4 injuries (see Fig. 138–3) (Wessells et al, 1997a; Moog et al, 2003). Currently, renal scans are obtained in two circumstances: when there is concern for long-term renal prognosis or in the presence of post-trauma–induced hypertension (Moog et al, 2003; Heyns, 2004). Classically, if the serum creatinine is normal, a differential renal function of greater than or equal to 30% demonstrates satisfactory ipsilateral renal function, which would prevent renal failure if the uninjured kidney is lost. In the presence of post-trauma–induced hypertension, mercaptoacetyltriglycine (mertiatide, MAG3) renography can be used to screen for trauma-induced renal vascular stenosis. Notably, the vast majority of patients with trauma-induced hypertension will have a kidney that has healed with a differential renal function of less than or equal to 20% (Wessells et al, 1997a; Moog et al, 2003; Heyns, 2004; Keller et al, 2004; Santucci et al, 2004b; Chedid et al, 2006) (Fig. 138–6).
Key Points
Radiographic and Endoscopic Assessment and Treatment of Pediatric Upper Tract Genitourinary Injuries
Management Of Renal Trauma
Multiple studies have stated that the nephrectomy rate in patients with traumatic renal injuries was higher with surgical exploration than with nonoperative management (Cass and Ireland, 1973; Cass et al, 1987; Kristjanson and Pederson, 1993; Hammer and Santucci, 2003; Keller et al, 2004; Broghammer et al, 2007). These papers suggested that hemorrhage from the severely injured kidney was held in check by a tamponade maintained by an intact Gerota fascia. Surgical exploration with disruption of the fascia resulted in uncontrollable renal bleeding and the need for emergent nephrectomy. Current studies reveal that this long-held hypothesis appears inaccurate. Specifically, with the enhanced ability of the CT scan to accurately stage renal injuries and with the development and application of trauma-related severity scores, multiple current studies reveal that the need for emergent nephrectomy during renal exploration is not due to intractable hemorrhage incited by the renal exploration; rather, emergent nephrectomy is usually due to either the severity of the initial renal injury and/or was performed in patients who had severe intraoperative hemodynamic instability due to multiple coexisting injuries. Nephrectomy in this latter situation was performed for expediency to save the hypothermic, coagulopathic, and clinically unstable patient (Husmann et al, 1993b; Wessells et al, 1997b; Gonzalez et al, 1999; Santucci et al, 2001, 2004b; Santucci and McAninch, 2001; Bozeman et al, 2004; Davis et al, 2006; Wright et al, 2006; Broghammer et al, 2007; Shariat et al, 2007, 2008).
Although all authorities agree that a clinically stable patient with an isolated renal injury should be managed in a nonoperative fashion, considerable controversy exists regarding the management of grade 3 or higher renal injuries when intra-abdominal injuries that mandate surgical exploration are found. There are three schools of thought in this controversy: (1) Provided no absolute indications for renal exploration are present, all renal trauma should be observed (Altiman et al, 2000; Hammer and Santucci, 2003; Keller et al, 2004); (2) renal exploration and renorrhaphy of a grade 3 or higher renal injury should be carried out if a laparotomy was to be performed for a coexisting intra-abdominal injury (especially if the stomach, duodenum, pancreas, or colon was injured) (Corriere et al, 1991; Husmann et al, 1993b; Heyns, 2004; Santucci et al, 2004b); and (3) renal exploration can be excluded in patients with concurrent intra-abdominal injuries, provided the trauma surgeon separates the site of the enteric injury from the urinary tract by omentum or other alternative tissue and places perioperative drains. It is hypothesized that the separation of the two sites of injury and the placement of drains will prevent breakdown of enteric repairs caused by leaking urine and/or help prevent the development of urinary tract complications by removal of excess contaminating bacteria or pancreatic enzymes from the site of GU injury (Husmann et al, 1993b; Wessells and McAninch, 1996; Matthews et al, 1997; El Khader et al, 1998; Santucci et al, 2004b; Broghammer et al, 2007). Due to the controversy, the major problem facing the urologist in the patient with traumatic renal injury is determining when to surgically intervene. The current recommendations on when to pursue operative intervention are based on three findings: the hemodynamic stability of the patient, an accurate radiographic staging of the renal trauma, and the presence of associated organ injury (Table 138–3) (Husmann and Morris, 1991; Husmann et al, 1993b; Wessells et al, 1997b; Heyns, 2004; Santucci et al, 2004b; Buckley and McAninch, 2006).
Table 138–3 Consensus Recommendations for Management of Renal Trauma
| CLINICAL FINDINGS AND/OR GRADE OF RENAL INJURY | RECOMMENDED TREATMENT |
|---|---|
| Grade 1 or 2 renal injury irrespective of traumatic etiology* | Nonoperative |
| Isolated grade 3, 4, and hemodynamically stable grade 5 renal injuries | Nonoperative |
| Uncontrollable renal hemorrhage/vascular instability (usually grade 4 vascular or grade 5 injuries) | Absolute requirement for surgical intervention |
| Persistent or delayed hemorrhage not responding to angiographic embolization | Absolute requirement for surgical intervention |
| Expanding pulsatile retroperitoneal mass found on surgical exploration for coexisting intra-abdominal injuries | Absolute requirement for surgical intervention (verify contralateral renal function prior to exploration) |
| Penetrating trauma, inadequate preoperative radiographic staging due to vascular instability of patient, retroperitoneal hemorrhage found on exploration | Retroperitoneal (renal) exploration recommended (verify contralateral renal function) |
| Blunt trauma, inadequate preoperative radiographic staging due to vascular instability of patient, retroperitoneal hemorrhage found on exploration | Retroperitoneal (renal) exploration recommended (verify contralateral renal function) |
| Blunt/penetrating trauma, radiographic screening studies reveal grade 3 with devitalized renal fragments, grade 4 or 5 renal injury, coexisting intra-abdominal injuries—especially, duodenum, pancreas, and colon | Retroperitoneal (renal) exploration with renorrhaphy and repair recommended |
Nonoperative Therapy for Renal Trauma
The ideal candidate for nonoperative management is the hemodynamically stable patient sustaining either blunt or penetrating trauma, with or without associated intra-abdominal injuries, and having a grade 1 to 2 renal injury. Genitourinary complications in this subset of patients are minimal (Wessells et al, 1997b; Heyns, 2004; Santucci et al, 2004b). Patients who are hemodynamically stable with isolated grade 3, 4, and 5 renal injuries are also candidates for nonoperative treatment. Even identification of a large perinephric hematoma or urinoma is not an absolute contraindication for nonoperative management, provided the distal ureter is documented to be intact (see Fig. 138–5). In patients with isolated grade 3 to 4 renal injuries, angiographic, endoscopic, or percutaneous intervention will be required in up to 55% of the patients. Intervention will be necessary for persistent or delayed bleeding in approximately 25%, symptomatic urinomas in approximately 25%, and surgical exploration to control complications not amenable to nonoperative techniques will develop in approximately 5%. In essence, conservative management of isolated grade 3 or 4 renal injuries will save approximately 95% of the patients by open surgical intervention (Husmann and Morris, 1991; Husmann et al, 1993b; El Khader et al, 1998; Bozeman et al, 2004; Buckley and McAninch, 2004, 2006; El-Sherbiny et al, 2004; Heyns, 2004; Santucci et al, 2004b; Broghammer et al, 2006, 2007; Henderson et al, 2007; Shariat et al, 2008).
Nonoperative therapy consists of bed rest, close monitoring of vital signs and urine output, serial abdominal examinations, serial hemoglobin/hematocrit determinations, and transfusion as indicated (Heyns, 2004; Santucci et al, 2004b). In patients with a renal injury secondary to penetrating trauma on a nonoperative protocol, intravenous broad-spectrum antibiotics are recommended, due to the risk of wound contamination. In renal injuries following blunt trauma, antibiotics may be considered if a large retroperitoneal hematoma, urinary extravasation, or extensive soft tissue injuries are present. The use of antibiotics following blunt trauma is advocated due to the presence of indwelling urethral catheters and/or multiple intravascular catheters or superficial dermatologic abrasions that could serve as a nidus for bacteremic colonization of the urine or blood and, hypothetically, result in an infected hematoma or urinoma (Husmann and Morris, 1991; Husmann et al, 1993b; Al-Qudah and Santucci, 2006; Buckley and McAninch, 2004, 2006; Heyns, 2004; Kansas et al, 2004; Santucci et al, 2004b).
If the child becomes hemodynamically unstable or continues to have a decreasing hemoglobin/hematocrit despite transfusions, renal angiography with selective angioinfarction of the bleeding site is recommended. If the patient develops a symptomatic urinoma, consideration for endoscopic or percutaneous management of the urinoma will be needed. Ambulation is allowed as soon as gross hematuria has resolved; resumption of strenuous physical activity should be avoided for 6 weeks. At 6 weeks, a physical examination and urinalysis are obtained; if the physical examination is normal and hematuria has cleared, the patient is allowed to resume all activities. Patients are reevaluated again at 12 weeks with a repeat physical examination and urinalysis, in addition to a repeat radiographic evaluation for patients with a grade 3 renal injury with devitalized fragments, grade 4, and grade 5 injuries (Wessells et al, 1997b; Heyns, 2004; Kansas et al, 2004; Santucci et al, 2004b; Al-Qudah and Santucci, 2006; Buckley and McAninch, 2006; Broghammer, 2007).
Operative Intervention for Renal Trauma
Absolute indications for renal exploration are hemodynamic instability due to a renal source, an expanding or pulsatile retroperitoneal hematoma, and inability to stop persistent or delayed hemorrhage by selective vascular embolization. Relative indications for exploration are a history of vascular instability resulting in an inability to obtain adequate preoperative radiographic evaluations, where surgical exploration for intra-abdominal injuries reveals a retroperitoneal hematoma. It is important to note that a single-shot IVP to verify contralateral renal function is required before renal exploration. Another relative indication for surgical exploration is the presence of coexisting intra-abdominal injuries associated with a grade 3 or higher renal injury (see Table 138–3) (Heyns, 2004; Santucci et al, 2004b). In this latter situation, the consensus opinion is that patients with a known grade 3 or higher renal injury undergoing exploratory laparotomy for multiorgan injury should undergo either renal exploration with renorrhaphy and the placement of retroperitoneal drains or separation of the urinary tract injury from adjacent enteric injuries by interposing omentum or other tissue with concurrent placement of adjacent drainage tubes. Although the surgeon can choose between the two options noted above, he or she should be aware that the consensus opinion is that renal exploration and renorrhaphy are preferred (Husmann et al, 1993b; Wessells et al, 1997b; Santucci and McAninch, 2001; Buckley and McAninch, 2004, 2006; Heyns, 2004; Santucci et al, 2004b).
Renal salvage by renorrhaphy or partial nephrectomy requires complete exposure of the injured kidney, debridement of nonviable tissue, suture ligation of bleeding arterial vessels, and repair of the collecting system injury. Defects in the renal parenchyma may be closed primarily with renal capsule. For larger defects, we prefer the placement of Gelfoam (Pfizer, New York, NY) and Surgicel (Ethicon, Somerville, NJ) packing into the parenchymal defect, with coverage or closure of the renal capsule defect using woven polyglycolic acid mesh. Alternatively, perinephric fat, omentum, or thrombin-soaked gel foam may be used to pack the parenchymal defect. Watertight closure of the collecting system is not always possible and may be inadvisable. If the renal pelvis or ureter is closed with excessive stretch, further devascularization, tissue sloughing, and delayed urinary leakage may occur. If a major violation of the urinary drainage system is present, placement of intraoperative ureteral stents or a nephrostomy tube should be considered. Adequate drainage of the perinephric area following repair is vital. In the presence of concurrent duodenal, pancreatic, and colonic injuries, interposition of omentum or peritoneum between the site of the major renal injury and the site of the coexisting intra-abdominal injury is strongly advised. Nephrectomy should be considered in irreparable grade 4 to 5 renal injuries and in the hemodynamically unstable patient with multiorgan trauma. A nephrectomy in this latter situation may need to be performed to reduce operative time and help control bleeding in the hypothermic and coagulopathic patient (Heyns, 2004; Santucci et al, 2004b; Davis et al, 2006; Wright et al, 2006; Broghammer et al, 2007; Shariat et al, 2008).
Key Points
Renal Trauma
Renal Vascular Injuries
In terms of arterial renal blood flow, the kidney is an end organ; only rarely is collateral blood flow outside of the main renal arterial supply sufficient to maintain renal function. In a patient sustaining renal arterial trauma, the clinical triad of hemodynamic instability, inadequate collateral blood flow, and warm ischemic time almost invariably results in the inability to salvage renal function (Heyns, 2004; Santucci et al, 2004a
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree