Germ cell tumors
Intratubular germ cell neoplasia, unclassified type (IGCNU)
Seminoma (including cases with syncytiotrophoblastic cells)
Spermatocytic seminoma (mention if there is sarcomatous component)
Embryonal carcinoma
Yolk sac tumour
Choriocarcinoma
Teratoma (mature, immature, with malignant component)
Tumours with more than one histological type (specify percentage of individual components).
Sex cord/gonadal stromal tumours
Leydig cell tumour
Malignant Leydig cell tumour
Sertoli cell tumour
lipid-rich variant
Sclerosing
Large cell calcifying
Malignant Sertoli cell tumour
Granulosa cell tumour (adult and juvenile types)
Thecoma/fibroma group of tumours
Other sex cord/gonadal stromal tumours (incompletely differentiated, mixed
Tumours containing germ cell and sex cord/gonadal stromal (gonadoblastoma).
Miscellaneous non–specific stromal tumours
Ovarian epithelial tumours
Tumours of the collecting ducts and rete testis
Tumours (benign and malignant) of non-specific stroma.
Serum Tumour Markers
Testicular cancer markers assist in diagnosis, staging, monitoring the treatment, recurrence detection and in the assessment of prognosis. They are measured prior to orchidectomy. Table 27.2 outlines the tumour markers that are useful in the management of testicular cancer. Pure seminomas do not have any specific markers although some have associated elevated levels of hCG. When seminomas are associated with raised AFP, these are considered and treated as non-seminoms. In NSGCTs the degree of tumour marker elevation after orchidectomy is considered as one of the predictors of prognosis [3].
Table 27.2
Tumour markers for testicular cancer
Marker | Association with types of testicular cancer | Other non-testicular tumours |
|---|---|---|
Alpha-fetoprotein (AFP) | Yolk sac cells | Gastrointestinal and hepatic tumours; nonmalignant conditions of liver |
Embryonal carcinoma | ||
Beta-chorionic gonadotrophin (hCG) | Choriocarcinoma, embryonal carcinoma | Gastrointestinal malignancies, lung, breast, kidney and bladder |
Some seminomas | ||
Lactic dehydrogenase (LDH) | Nonspecific for GCTs | Muscle injury, some lymphomas |
Indicate bulkiness of the tumour |
Intratubular Germ Cell Neoplasia (ITGCN, Carcinoma In Situ CIS)
Testicular carcinoma in situ is known as the precursor of germ cell cancers of the testis [4] with the exception of spermatocytic seminoma seen commonly in older men, and infantile tumours (yolk sac tumor and mature teratoma) [5]. There is a substantial body of evidence to suggest that ITGCN is a precursor of testicular germ cell tumors (TGCTs) during intrauterine life, remaining dormant until the age of puberty. Thereafter, under the influence steroids and gonadotropins and environmental and lifestyle factors, ITGCN becomes an invasive seminomatous or nonseminomatous germ cell tumor [6]. The risk of ITGCN is also increased in the contralateral testis of patients with testicular carcinoma [7]. It is believed that ITGCN originates from gonocytes [8].
ITGCN is likely to develop into invasive malignancy in 50 % of patients within 10 years and 70 % within 7 years [7] and the most common type is a seminoma [9]. The cells of ITGCN have a number of similarities to embryonic germ cells, such as their positivity for alkaline phosphatase, increased glycogen content, and the presence of stem cell factor receptor (c-KIT). Based on immunohistological findings, Jørgensen et al. [10] have suggested that the cell of origin of ITGCN is present in the gonad around the 7th to 10th week of intrauterine life. In 5 % of cases with unilateral seminoma or nonseminoma there is a contralateral ITGCN, indicating major events taking place even before primordial germ cells reach the left and right gonadal blastema [11]. Known risk factors for ITGCN include contralateral testicular malignancy, cryptorchidism, atrophic testis, infertility and true hermaphroditism [12].
Histopathological Features
The diagnosis of ITGCN requires testicular biopsy and the specimen should be placed in Bouin’s or Stieve’s solution, as formalin causes shrinkage artifacts. The cells are larger than normal spermatogonia with abundant cytoplasm and hyperchromatic nuclei, which have prominent nucleoli (Table 27.3).
Larger than normal spermatogonia |
Clear or vacuolated cytoplasm rich in glycogen |
Nuclei: large, irregular, and hyperchromatic |
Nucleoli: one or more large and irregular |
Abnormal mitoses |
Basally located cells |
Spermatogenesis commonly absent |
Segmental involvement of tubules |
The cell margins are conspicuous and cytoplasm is abundant and the basement membrane is frequently thickened [14]. ITCGN is seen within seminiferous tubules surrounding invasive carcinomas in the majority of cases [15] (Fig. 27.1).
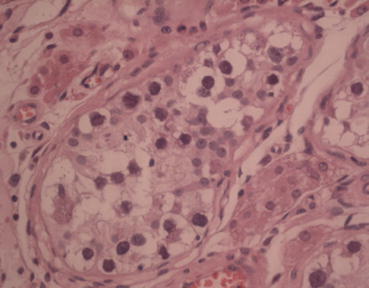

Fig. 27.1
Testicular carcinoma in situ (H & E ×40)
A wide variety of histological markers are used in the diagnosis of ITGCN. The most widely used marker is placental alkaline phosphatase [16]. Another most specific recent marker is Oct¾ [17]. They are also associated with positive CD117 [18].
Seminoma
Seminoma is the most common germ cell tumour accounting for almost half of all cases [19]. Patients with seminoma typically present between the ages of 35–45 years and the majority of patients present with testicular enlargement [1]). Macroscopically, seminomas are usually well-circumscribed, firm and homogenous tumours.
Microscopically (Fig. 27.2), the tumour is composed of sheets of large monomorphic cells with copious clear cytoplasm, a definite cell membrane and a single nucleus with prominent nucleoli. Thin fibrous septa are present which divides the tumour into lobules. Numerous lymphocytes are typically present and a granulomatous reaction can often be seen. In a proportion of cases, syncytiotrophoblastic cells can be observed scattered through the tumour [20]. A seminoma is typically positive for PLAP, OCT4 [21] and CD117 [22]. In cases where syncytiotrophoblastic giant cells are present within the tumour, these cells can be highlighted using immunohistochemistry for human chorionic gonadotrophin (HCG) [23]. The most common cytogenetic abnormality in seminoma is the presence of the isochromosome 12p [24].
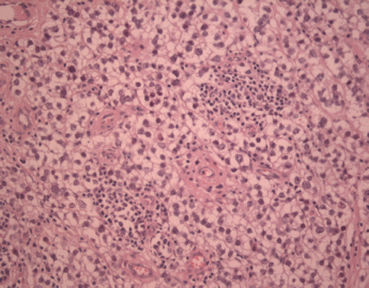

Fig. 27.2
Seminoma (H&E ×40)
Spermatocytic Seminoma
This is an uncommon germ cell tumour which typically occurs in men over the age of 50 years and demonstrates indolent clinical behaviour. Patients typically present with testicular enlargement [25]. Macroscopically, the tumour is soft and friable [25]. Microscopically, the tumour is composed of three cell types: large, medium-sized and small cells. The large cells have copious cytoplasm and can be either single or multinucleate. The nuclei are usually round but can show size variation. The medium-sized cells have round nuclei with granular cytoplasm and eosinophilic cytoplasm. The small cells have a similar appearance to lymphocytes.
Spermatocytic seminomas are not associated with other neoplastic germ cell components or with classical seminoma [25]; however a small percentage of cases can be associated with high-grade sarcoma. The presence of high-grade sarcoma correlates with aggressive clinical course [26]. The immunohistochemical markers, PLAP and OCT4, which are positive in classical seminoma are negative in spermatocytic seminoma [27]. CD117 is typically positive in spermatocytic seminoma [28].
Nonseminomatous Germ Cell Tumors
These tumors have embryonic elements and differentiate along embryonic lineage (embryonal carcinoma, teratoma, teratocarcinoma) or extraembryonic tissue components (yolk sac tumor and choriocarcinoma).
Choriocarcinoma
It is a rare but highly aggressive tumour that typically occurs in young men in the second and third decade. It is rarely presents in a pure form [19] however, it is often found as a component of NSGCT. Patients often present with a raised serum hCG level and symptoms of metastatic disease [1]. Macroscopically, the tumour has a necrotic haemorrhagic appearance. Microscopically, the tumour is composed of syncytiotrophoblasts and cytotrophoblasts usually arranged in solid sheets [29]. Syncytiotrophoblastic cells are large multinucleated giant cells with dark eosinophilic cytoplasm; cytotrophoblasts are medium sized polygonal cells with clear cytoplasm. These cells can be difficult to distinguish from embryonal carcinoma cells or seminoma cells, however their proximity to syncytiotrophoblastic cells helps identify them. Syncytiotrophoblastic cells often wrap around the cytotrophoblast and form villous structures [1]. Immunohistochemistry demonstrates the syncytiotrophoblastic tumour cells are strongly positive for HCG, cytokeratin is positive in both syncytiotrophoblastic and cytotrophoblastic cells [29].
Yolk Sac Tumour (Endodermal Sinus Tumour)
The pure form of yolk sac tumour is rarely seen in adults and is usually seen admixed with other germ cell tumours. Patients often present with high serum alpha-fetoprotein (AFP) [30] and rapid testicular enlargement [1]. Yolk sac tumour is a common tumour of infants, more than 75 % of testicular tumours in childhood are yolk sac tumours and are typically found in the pure form [31]. Macroscopically, the testis contains a poorly defined lobulated tumour which may contain cystic areas or areas of haemorrhage and necrosis. Microscopically, the tumour can demonstrate numerous patterns with the most common being a reticular-microcystic pattern (Fig. 27.3). This is composed of anatomizing channels lined by cuboidal cells forming loose cystic spaces. The microcystic pattern contains small cystic spaces with clusters of cells projecting into the lumen. Several other patterns are also seen but with reduced frequency. The definitive feature of YST is the formation of Schiller-Duval bodies, these are fibrovascular cores surrounded by malignant cells, which are set within a cystic space lined by flat epithelial cells. Intracellular hyaline globules are also characteristic of yolk sac tumour [1]. immunohistochemistry demonstrates the tumour cells are positive for AFP [30] and CEA, and are negative for HCG [32].
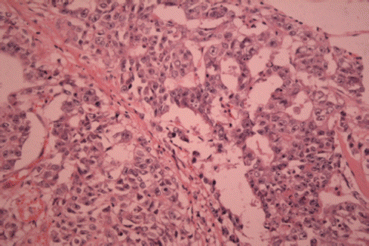

Fig. 27.3
Embryonal cell carcinoma (H & E ×40)
Embryonal carcinoma. It comprises approximately 15–30 % of pure testicular germ cell neoplasms and typically occurs in patients between the ages of 15–34 years. The majority of patients have metastatic disease at presentation with half have distant metastases. Despite the inherent tumour aggressiveness overall 5-year survival rate is over 80 % although survival correlates with disease extent at diagnosis [33].
Macroscopically, embryonal carcinoma is often a small tumour which is poorly circumscribed and contains areas of haemorrhage and necrosis. One fifth of embryonal carcinomas show extension beyond the testis at the time of operation. Microscopically, embryonal carcinoma can demonstrate solid, tubular or papillary patterns with areas of necrosis or haemorrhage. The cells are large and pleomorphic with large irregular nuclei, abundant cytoplasm and numerous mitoses.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







