Jonathan I. Epstein, MD
Prostatic Intraepithelial Neoplasia
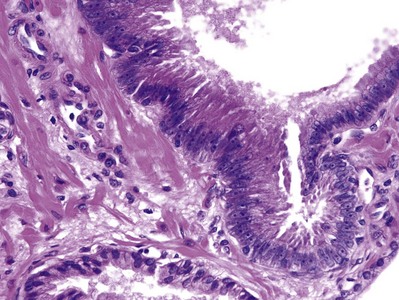
Prostatic intraepithelial neoplasia (PIN) consists of architecturally benign prostatic acini or ducts lined by cytologically atypical cells and is classified as low-grade and high-grade neoplasias (McNeal and Bostwick, 1986) (Fig. 96–1). Diagnostic reports should not comment on low-grade PIN. First, pathologists cannot reproducibly distinguish between low-grade PIN and benign prostate tissue (Epstein et al, 1995). Second, when low-grade PIN is diagnosed on needle biopsy, these patients are at no greater risk of having carcinoma on repeated biopsy than are men with a benign biopsy finding (Epstein and Herawi, 2006). Evidence that high-grade PIN (HGPIN) is a precursor to some prostate carcinomas includes the following: There is an increase in the size and number of high-grade PIN foci in prostates with cancer compared with prostates without carcinoma; with increasing amounts of high-grade PIN, there are a greater number of multifocal carcinomas; both high-grade PIN and carcinoma preferentially involve the peripheral zone; and biomarkers and molecular changes show similarity between high-grade PIN and carcinoma (Bostwick et al, 1996; Haggman et al, 1997). About 20% of high-grade PIN lesions harbor a TMPRSS2-ERG fusion gene, which is a common molecular abnormality detectable in about 50% of prostate cancers (Cerveira et al, 2006; Perner et al, 2007).
The incidence of high-grade PIN on biopsy averages 7.6% among 38 studies, with a median of 5.2%. There is a tremendous variation in the percentages reported, ranging from 0% to 25%, with no apparent correlation to number of cores sampled or whether the study was from an academic or community hospital setting (Epstein and Herawi, 2006). The most likely explanation accounting for this variation is interobserver threshold. The distinction between low-grade and high-grade PIN is based on the prominence of the nucleoli. This is a subjective exercise, and those pathologists with a lower threshold as to what defines prominent nucleoli will have a higher incidence of high-grade PIN. The largest studies reported a 16% to 44.6% risk of cancer on subsequent biopsy. Of the 11 studies with at least 50 cases of high-grade PIN on needle biopsy with follow-up, the mean risk of cancer is 26.4% (Epstein and Herawi, 2006). In the majority of studies, serum prostate-specific antigen (PSA) levels, results of digital rectal examination, and transrectal ultrasonography findings do not enhance the prediction of who is more likely to have carcinoma on repeat biopsy. PIN by itself does not give rise to elevated serum PSA values (Ronnett, et al, 1993). Of 8 studies in which the risk of cancer after a needle biopsy diagnosis of high-grade PIN was compared with the risk of cancer after a benign biopsy finding, 6 showed no difference between the groups (Epstein and Herawi, 2006). For patients diagnosed with unifocal high-grade PIN on extended initial core sampling, a repeat biopsy within the first year is unnecessary in the absence of other clinical indicators of cancer and in the absence of extensive high-grade PIN. High-grade PIN on greater than or equal to two cores is associated with a sufficiently high risk of subsequent cancer that rebiopsy within a year of the initial PIN diagnosis is warranted (Abdel-Khalek et al, 2004; Netto and Epstein, 2006; Merrimen et al, 2009). Lefkowitz and colleagues (2002) described 31 men who had an initial diagnosis of high-grade PIN on 12-core biopsy with an interval to follow-up prostate biopsy of 3 years. The rate of cancer on repeat 12-core biopsy was 25.8% compared with their earlier study in which rebiopsy within 1 year of a diagnosis of high-grade PIN yielded cancer in only 2.3%. They hypothesized that the 3-year interval allowed unsampled small cancers that were associated with the high-grade PIN at the time of initial biopsy to grow to a size that was detectable on repeat biopsy, or alternatively, some of the high-grade PIN lesions progressed to cancer during the 3-year interval. If a repeat prostate needle biopsy is performed, it should sample the entire prostate and not just the initial sextant site where the high-grade PIN was found (Epstein and Herawi, 2006).
The significance of HGPIN on transurethral resection (TUR) is not clear, with conflicting data as to the risk for subsequent discovery of cancer (Gaudin et al, 1997; Pacelli and Bostwick, 1997). In an elderly patient with HGPIN on TUR, often no further workup is instituted. In a younger man, a more aggressive workup to rule out a clinically significant tumor is warranted. The one piece of evidence we have for premalignant lesions in other organs that is lacking in the prostate is the natural history of high-grade PIN. With the prostate, there is no capability to monitor a PIN focus to determine whether there is already infiltrating carcinoma at that site or whether infiltrating carcinoma has evolved in the immediate vicinity of the PIN focus. Because we do not know what percentage of patients develop infiltrating carcinoma during a given follow-up interval, when high-grade PIN is found on biopsy material, most authorities do not use the term carcinoma in situ of the prostate. It appears that high-grade PIN is a precursor lesion to many peripheral intermediate- to high-grade adenocarcinomas of the prostate. However, PIN need not be present for carcinoma to arise. Low-grade carcinomas, especially those present within the transition zone, are not closely related to high-grade PIN.
Intraductal carcinoma of the prostate (IDC-P) has, in several studies, been described in radical prostatectomy specimens (McNeal et al, 1986; McNeal and Yemoto, 1996; Rubin et al, 1998; Wilcox et al, 1998; Cohen et al, 2007; Robinson and Epstein, 2010). Rarely, IDC-P may be identified on biopsy material in the absence of infiltrating carcinoma (Guo and Epstein, 2006). IDC-P on prostate biopsies is frequently associated with high-grade cancer and poor prognostic parameters at radical prostatectomy, as well as potentially advanced disease following other therapies (Guo and Epstein, 2006). These findings support the idea that in most cases IDC-P represents intraductal spread of carcinoma within pre-existing ducts and acini and that IDC-P in the vast majority of cases should not be categorized as a preinvasive neoplastic condition. Consideration should be given to aggressively treating patients with IDC-P on biopsy, even in the absence of documented infiltrating cancer.
Adenocarcinoma
Location
In clinical stage T2 carcinomas and in 85% of nonpalpable tumors diagnosed on needle biopsy (stage T1c), the major tumor mass is peripheral in location (McNeal, 1969; Byar and Mostofi, 1972; Epstein et al, 1994b). In the remaining cases, tumors are predominantly located in the transition zone (i.e., periurethrally or anteriorly). Tumors that appear to be unilateral on rectal examination are bilateral in approximately 70% of cases when they are examined for pathology. Adenocarcinoma of the prostate is multifocal in more than 85% of cases (Byar and Mostofi, 1972). In many of these cases of bilateral or multifocal tumor, the other tumors are small, low grade, and clinically insignificant. Consequently, the distinction between pathologic stages T2a and T2b is meaningless (Freedland et al, 2004). In a highly selected population with limited unilateral biopsy cancer, the mean number of separate tumor nodules per radical prostatectomy is 2.9. A contralateral tumor to the positive biopsy side at radical prostatectomy is typically small. However, 20% have some contralateral adverse pathology in terms of size, extraprostatic extension, grade, or margins (Yoon et al, 2008).
Spread of Tumor
Because the prostate lacks a discrete histologic capsule, extraprostatic extension is preferable to “capsular penetration” as the term to describe a tumor that has extended out of the prostate into periprostatic soft tissue (Ayala et al, 1989). Some authors use the term capsular invasion when they believe that the “capsule” is infiltrated by a tumor but the tumor does not extend out of the prostate. Because there is no such entity as the prostatic capsule, “capsular invasion” makes no sense. Peripherally located adenocarcinomas of the prostate tend to extend out of the prostate through perineural space invasion (Villers et al, 1989). Perineural invasion by itself in radical prostatectomy specimens does not worsen prognosis, because perineural invasion merely represents extension of a tumor along a plane of decreased resistance and not invasion into lymphatics (Hassan and Maksem, 1980). In contrast, vascular invasion increases the risk of recurrence after radical prostatectomy (Baydar et al, 2008). Extraprostatic extension preferentially occurs posteriorly and posterolaterally, paralleling the location of most adenocarcinomas.
Further local spread of tumor may lead to seminal vesicle invasion, which is diagnosed when a tumor extends into the muscle wall of the seminal vesicle. The most common route of seminal vesicle invasion is by tumor penetration out of the prostate at the base of the gland, with growth and extension into the periseminal vesicle soft tissue and eventually into the seminal vesicles. Less commonly, there may be direct extension through the ejaculatory ducts into the seminal vesicles or direct extension from the base of the prostate into the wall of the seminal vesicles. Least commonly, there may be discrete metastases to the seminal vesicle (Fry et al, 1979; Ohori et al, 1993). Local spread of prostate cancer may also rarely involve the rectum, where it may be difficult to distinguish from a rectal primary tumor (Fry et al, 1979; Lane et al, 2008).
The most frequent sites of metastatic prostate carcinoma are lymph nodes and bone. Prostate cancer may present with metastases to the left supradiaphragmatic, typically the supraclavicular, lymph nodes (Cho and Epstein, 1987). Lung metastases from prostate carcinoma are extremely common at autopsy, and almost all cases have bone involvement as well (Varkarakis et al, 1974). Metastatic lesions usually take the form of multiple small nodules or diffuse lymphatic spread rather than large metastatic deposits. Clinically, prostate carcinoma metastatic to the lung is usually asymptomatic. In addition to lymph nodes, bones, and lung, the next most common regions of spread of prostate cancer at autopsy are bladder, liver, and adrenal gland (Hess et al, 2006).
Tumor Volume
In general, the size of a prostate cancer correlates with its stage. Extraprostatic extension is uncommon in tumors of less than 0.5 cm3, and tumors that are less than 4 cm3 uncommonly reveal lymph node metastases or seminal vesicle invasion (McNeal et al, 1990). Tumor volume is also proportional to grade (see following discussion). The location and grade of the tumor also modulate the effect of tumor volume (Christensen et al, 1990; McNeal et al, 1990; Greene et al, 1991). For example, transition zone tumors extend out of the prostate at larger volumes than do peripheral zone tumors, because of their lower grade and greater distance from the edge of the gland.
Grade
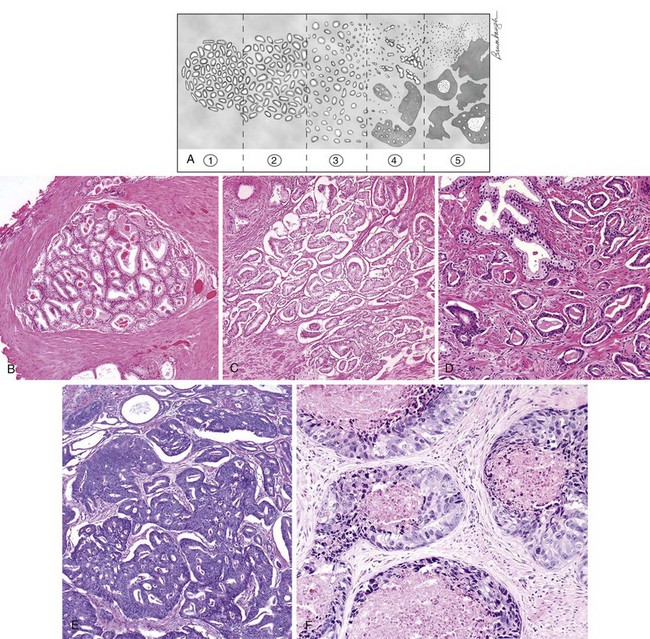
Although numerous grading systems exist for the evaluation of prostatic adenocarcinoma, the Gleason grading system is the most widely accepted (Gleason and Mellinger, 1974). The Gleason system is based on the glandular pattern of the tumor as identified at relatively low magnification (Fig. 96–2). Cytologic features play no role in the grade of the tumor. Both the primary (predominant) and the secondary (second most prevalent) architectural patterns are identified and assigned a grade from 1 to 5, with 1 being the most differentiated and 5 being the least differentiated (Table 96–1). Because both the primary and the secondary patterns are influential in predicting prognosis, there is a Gleason sum or score obtained by the addition of the primary and secondary grades. If a tumor has only one histologic pattern, then for uniformity, the primary and secondary patterns are given the same grade. Gleason scores range from 2 (1 + 1 = 2), which represents tumors uniformly composed of Gleason pattern 1 tumor, to 10 (5 + 5 = 10), which represents totally undifferentiated tumors. This author prefers to assign both a primary and a secondary pattern even when presented with limited cancer so as to not to create any confusion. For example, cases that the pathologist signs out as only “Gleason grade 4” could be interpreted to mean Gleason pattern 4 (high-grade cancer) or Gleason score 4 (low-grade cancer). In radical prostatectomy specimens, it has been demonstrated that tertiary (third most common pattern) high-grade components adversely affect biologic behavior, yet are not always equivalent to the sum of the primary pattern and highest-grade pattern. It is recommended that in radical prostatectomy specimens, the routine Gleason score, consisting of the most prevalent and the second most prevalent architectural patterns, be recorded along with a note stating that there is a tertiary high-grade pattern (Pan et al, 2000; Trock et al, 2009). There are a few exceptions to the Gleason system, as described above. For needle biopsy specimens in which the typical scenario includes tumors with patterns 3, 4, and 5 in various proportions, both the primary pattern and the highest grade should be added to derive the Gleason score. Any amount of high-grade tumor sampled on needle biopsy most likely indicates a more significant amount of high-grade tumor within the prostate because of the correlation of grade and volume and the problems inherent with needle biopsy sampling. In the setting of high-grade cancer, one should ignore lower-grade patterns if they occupy less than 5% of the area of the tumor. For example, a tumor composed of 98% Gleason pattern 4 and 2% Gleason pattern 3 should be diagnosed as Gleason score 4 + 4 = 8 (Epstein et al, 2005).
Table 96–1 2005 International Society of Urological Pathology Modified Gleason System
| Pattern 1 |
| Pattern 2 |
Like pattern 1, fairly circumscribed, yet at the edge of the tumor nodule there may be minimal infiltration |
| Pattern 3 |
| Pattern 4 |
| Pattern 5 |
It is important to recognize Gleason pattern 4 tumors because tumors with this pattern have a significantly worse prognosis than those with pure Gleason pattern 3 (McNeal et al, 1990; Epstein et al, 1993b). It has also been demonstrated in radical prostatectomy specimens that tumors with Gleason score 4 + 3 = 7 have a worse prognosis than those with Gleason score 3 + 4 = 7 (Chan et al, 2000). There is fairly good interobserver reproducibility of the Gleason system among uropathology experts and poorer reproducibility among practicing pathologists (Allsbrook et al, 2001a, 2001b). It has been demonstrated that although current use of the Gleason grading system is not optimal, significant improvements can be made after participation in relatively brief educational programs, such as those available on websites (e.g., www.isuporg.org [International Society of Urological Pathology]).
The Gleason grade on biopsy material has also been shown to correlate fairly well with that of the subsequent prostatectomy specimen (Fine and Epstein, 2008). Several studies have demonstrated that there is better correlation between the biopsy and prostatectomy grade with extended as opposed to sextant needle biopsy sampling. In general, a Gleason score less than or equal to 6 or greater than or equal to 7 on biopsy corresponds to a Gleason score less than or equal to 6 or greater than or equal to 7 in the radical prostatectomy, respectively, in 80% of cases. An unavoidable cause of discordant grading between the biopsy and subsequent prostatectomy specimen(s) concerns sampling errors with the needle biopsy. One of the most frequent causes of discordant grading is grading of tumors that straddle two grades. One way the practice of Gleason scoring can be improved is by virtually never assigning Gleason score 2 to 4 for adenocarcinoma of the prostate on needle biopsy. The reasons for this approach are as follows: (1) most tumors graded as Gleason score 2 to 4 on needle biopsy are graded as Gleason score 5 to 6 or higher when reviewed by uropathology experts (Steinberg et al, 2005); (2) there is poor reproducibility in the diagnosis of Gleason score 2 to 4 on needle biopsy even among uropathology experts (Allsbrook et al, 2001b); and (3) most important, assigning Gleason score 2 to 4 to an adenocarcinoma on needle biopsy can adversely affect the patient’s care, because clinicians may incorrectly assume that all low-grade cancers on needle biopsy do not need definitive therapy. Although low volume, Gleason score 2 to 4 adenocarcinoma of the prostate, on transurethral resection of the prostate, has a relatively indolent course; low-grade cancer on needle biopsy does not. Pathologists, in general, are less frequently overdiagnosing Gleason scores 2 to 4 on biopsy in recent years. In one study, 24% of pathologists rendered a diagnosis of Gleason score 2 to 4 on biopsy in 1991, which decreased to 2.4% in 2001 (Ghani et al, 2005).
The ultimate value of any grading system is its prognostic ability. Both Gleason’s data with 2911 patients and subsequent studies with long-term follow-up have demonstrated a good correlation between the Gleason sum and prognosis (Mellinger, 1977; Sogani et al, 1985). When stage of disease is factored in with grade, prognostication is enhanced. Some men with low-grade cancers develop high-grade tumors after several years (Brawn, 1983). It is not clear whether the residual low-grade cancer progressed or whether there was a subsequent development of a multifocal, more aggressive tumor. Although, in general, larger tumors are high grade and small tumors are low grade, exceptions occur (Epstein et al, 1994a). There is a tendency to hypothesize that tumors begin as low-grade tumors and, on reaching a certain size, dedifferentiate into higher-grade lesions, accounting for the relationship between size and grade. Alternatively, high-grade tumors may be high grade at their inception but are detected at an advanced size because of their rapid growth. Similarly, low-grade tumors may evolve so slowly that they tend to be detected at lower volumes. During a 2- to 3-year period after biopsy, there is no evidence that prostate cancer grades worsen significantly (Sheridan et al, 2008).
Assessment of Needle Biopsy Specimens
Processing
When biopsy specimens are taken from different areas of the prostate, they should be submitted to pathology in separate containers (Table 96–2). As long as cores are submitted in separate containers or the cores are in the same container yet specified by the urologist as to their location (i.e., by different color inks), pathologists should assign individual Gleason scores to separate cores (Epstein et al, 2005). If cores are combined in a container, one can try to give separate scores for each core or can give an overall Gleason score. For example, in a case with Gleason score 4 + 4 = 8 on one core and with pattern 3 (3 + 3 = 6, 3 + 4 = 7, 4 + 3 = 7) on other cores in the same container, the overall score for that container, averaging all involved needle biopsy specimens together as if they were one long positive core, would be Gleason score 4 + 3 = 7 or 3 + 4 = 7, depending on whether pattern 4 or 3 predominated.
Table 96–2 Reasons to Submit Needle Cores in Separate Jars for Each Sextant Site
• In “atypical” cases, the atypical site can be preferentially targeted on repeated biopsy, in addition to other sites. |
Differential Diagnosis
The underdiagnosis of limited adenocarcinoma of the prostate on needle biopsy is one of the most frequent problems in prostate pathology (Epstein, 2004). There are also numerous benign mimickers of adenocarcinoma of the prostate (Srigley, 2004). In some of these cases, the use of antibodies to high-molecular-weight cytokeratin and p63 may resolve the diagnosis (Wojno and Epstein, 1995). Benign glands contain basal cells and are labeled with these antibodies, whereas prostate cancer shows no staining. Immunohistochemistry with antibodies to α-methylacyl-CoA racemase, which preferentially labels prostatic carcinoma and high-grade PIN, can also be used as an adjunct in the diagnosis of limited cancer, yet pathologists must be careful because false-positive and false-negative staining with α-methylacyl-CoA racemase has been reported (Jiang et al, 2004).
In certain cases, there are findings suggestive of but not diagnostic of carcinoma. The incidence of atypical needle biopsy specimens reported from the 39 studies in the literature averages 7.6% with a median of 5.2% (Epstein and Herawi, 2006
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree