Pancreaticobiliary Surgery: General Considerations
Steven C. Cunningham
Aram N. Demirjian
Richard D. Schulick
Surgically Relevant Anatomy
The pancreas is a large, asymmetric gland lying in the central retroperitoneum, consisting of the head, neck, body, and tail. The pancreatic head lies at just right of the L2 vertebral body and extends in an oblique course to the left over the spine, cephalad, and then slightly posterior until the tail terminates near the splenic hilum, at the level of T10. The neck is often defined as that portion of the gland overlying the superior mesenteric artery and vein (SMA and SMV), and separating the head to the right from the body to the left. The dividing line between the body and tail hardly exists and is not surgically relevant. The uncinate process of the pancreas is embryologically separate from the rest of the pancreas and in adults extends from the inferior lateral head of the gland, extending slightly posterior to the SMV and terminating at the SMA (Fig. 1.1).
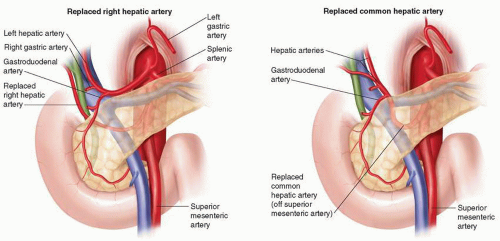
The arterial supply to the pancreas is abundant and comes via multiple named and unnamed vessels from both the celiac axis and the SMA, a fact largely responsible for the ability of the pancreaticoduodenectomy resection specimen to bleed abundantly until the last fibers of tissue are divided. The head is richly supplied by anastomosing branches of the pancreaticoduodenal arteries, while the body and tail are predominantly supplied by branches of the splenic artery and jejunal branches. The collateral flow often present between the SMA and the celiac axis, chiefly through the gastroduodenal artery (GDA), becomes very important at pancreaticoduodenectomy, during which the GDA is typically divided. In all cases, flow in the hepatic artery is confirmed during clamping of the GDA to detect cases in which hepatic artery flow is significantly dependent on SMA-celiac axis collaterals. In such cases, arterial bypass, preservation of the GDA, or division of a median arcuate ligament may be necessary, depending on the clinical scenario. In all cases, one must be aware of aberrant hepatic arterial anatomy (vida infra), which is common (>25% of cases) and is more commonly replaced than accessory (Fig. 1.2).
The venous drainage of the pancreas is predominantly portal, excepting small unnamed retroperitoneal veins that may drain posteriorly into the lumbar system and
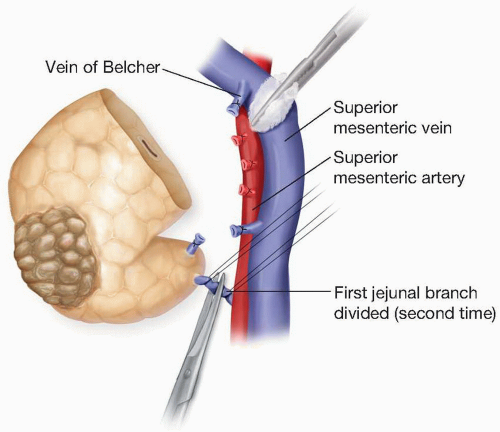
may in cases of portal hypertension become clinically relevant. The predominantly portal drainage of the pancreas accounts for the preponderance of liver metastases compared with lung metastases in cases of advanced pancreatic cancer. The pancreatic head and uncinate process drain via pancreaticoduodenal veins that run with the pancreaticoduodenal arteries and drain into the SMV and portal vein, while the body and tail drain via the splenic vein. During pancreaticoduodenectomy several prominent named veins must be ligated and divided at their confluence with the SMV in order to dissect the neck of the pancreas from the SMV. These include the gastroepiploic vein caudal and to the left, and the vein of Belcher, cephalad and to
the right. After division of the neck of the pancreas, a first jejunal vein is sometimes ligated and divided at its confluence with the SMV during division of the uncinate process (Fig. 1.3).
may in cases of portal hypertension become clinically relevant. The predominantly portal drainage of the pancreas accounts for the preponderance of liver metastases compared with lung metastases in cases of advanced pancreatic cancer. The pancreatic head and uncinate process drain via pancreaticoduodenal veins that run with the pancreaticoduodenal arteries and drain into the SMV and portal vein, while the body and tail drain via the splenic vein. During pancreaticoduodenectomy several prominent named veins must be ligated and divided at their confluence with the SMV in order to dissect the neck of the pancreas from the SMV. These include the gastroepiploic vein caudal and to the left, and the vein of Belcher, cephalad and to
the right. After division of the neck of the pancreas, a first jejunal vein is sometimes ligated and divided at its confluence with the SMV during division of the uncinate process (Fig. 1.3).
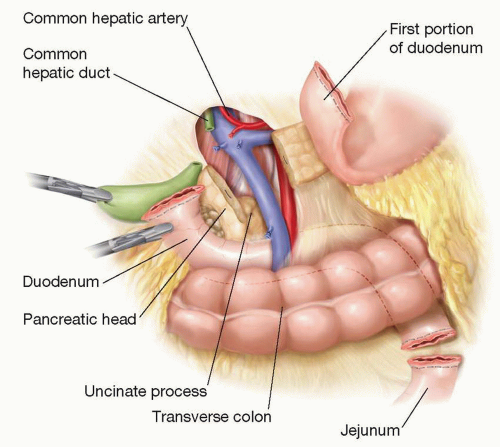 Figure 1.1 Illustration showing the relationship of the divided duodenum, common hepatic duct, jejunum, and pancreas to its surrounding structures. |
The vascular anatomy of the pancreas and surrounding structures is crucial to the surgical care of pancreaticobiliary patients, and in fact, often determines resectability of malignant masses. Typically, patients without metastatic disease are grouped into three categories of resectability, depending on the vascular involvement by the tumors: Resectable, borderline, and unresectable. Borderline patients were first defined by the MD Anderson group and may have either encasement of a short segment of the hepatic artery (but no extension to the celiac axis) that is amenable to resection and reconstruction, or tumor abutment (viz, <180 degrees) of the SMA, or short-segment occlusion of the SMV or portal vein that is amenable to resection and reconstruction. Tumor encasement (viz, ≥180 degrees) of the SMA by the tumor typically constitutes a locally advanced, unresectable tumor.
Pancreatic lymphatic vessels travel from the acini and follow the arteries to drain into peripancreatic lymph nodes. The head and neck of the pancreas drain widely into pancreaticoduodenal nodes, superior mesenteric nodes, hepatic artery nodes, pre-aortic, and celiac axis nodes. The body and tail drain predominantly into pancreaticosplenic nodes with a minority of channels draining into pre-aortic nodes. The pancreatic islets have no lymphatics.
The exocrine pancreas is richly innervated with both sympathetic and parasympathetic fibers (the endocrine pancreas is innervated almost exclusively by the parasympathetic system). Sensory fibers from the pancreas travel through the celiac plexus, at which point they are available for ablation, typically via ethanol splanchnicectomy in cases of severe, chronic pain from pancreatitis or locally advanced malignancy.
The extrahepatic biliary tree, as well as associated arteries, is aberrant in one way or another at least as often as they are typical. Although discussion of all the variations of the hepatic, cystic, and common bile ducts, as well as the cystic and hepatic arteries, is beyond the scope of this chapter, aberrancies of the right and left hepatic arteries are worthy of further mention. The most reliable measurement of the frequency and type of hepatic artery aberrancies likely comes from autopsy studies in the 1950s by Michels, who described in a series of 200 autopsies, that 26% of bodies had aberrant right, and 27% aberrant left, hepatic arteries. On both sides, replaced was more common than accessory arteries (60% replaced on the right and 70% on the left). The practicing pancreaticobiliary surgeon must be familiar with standard as well as aberrant anatomy of the extrahepatic biliary tree and associated arteries (Fig. 1.2).
Preoperative Considerations
Diagnosis
Patients with pancreaticobiliary disease present in a variety of ways. In cases of extrahepatic biliary tree pathology, and processes involving the head of the pancreas, this often manifests as painless jaundice. Abdominal pain, especially in cases of pancreatitis or bile duct strictures, can also occur frequently. Regardless of the presentation, once pancreaticobiliary disease has been identified, appropriate preoperative planning is a cornerstone of a successful surgical result, and multiple tools exist.
Imaging
Computerized Tomography
Computerized tomography (CT) scan is one of the most reliable planning implements in the preoperative phase, providing information not only regarding the size and location of the tumor, but also more importantly about its relationship to surrounding structures. Pancreatic adenocarcinomas usually appear as hypoattenuating lesions when the pancreatic parenchyma is maximally enhanced in the early postarterial phase. CT has very high sensitivity, approaching 100%, for lesions ≥2 cm in size. There does, however, appear to be a significant decrease for smaller tumors, with sensitivities ranging from 67% to 77%. CT is a powerful tool with regard to determining resectability, as sensitivity for vascular involvement can exceed 90%. Despite continuing advances in CT technology, one drawback remains the lack of accuracy in identifying small liver metastases, small peritoneal nodules, or low-volume carcinomatosis. This can be a significant issue, and can lead to a false negative result in >30% of cases.
Endoscopic Ultrasound
The role of endoscopic ultrasound (EUS) in the preoperative planning for pancreaticobiliary surgery is somewhat controversial. Like CT, EUS can provide information regarding the relationship of lesions to major vascular structures, but multi-detector CT scanning is of such high quality, that EUS is generally not necessary to accomplish this. Based on one study, sensitivity for EUS in detecting vascular invasion is 86%, while specificity is only 71%. EUS does, however, provide an excellent avenue for obtaining a tissue diagnosis. This may be necessary in patients with ambiguous lesions in order to determine if they should undergo pancreaticoduodenectomy, but is particularly important in marginally resectable or unresectable patients who will require chemotherapy or chemoradiotherapy. In addition, EUS may be the procedure of choice for identifying nodularity in patients with small pancreatic cysts. Lastly, EUS may be useful on occasion for identifying patients with metastatic disease, such as to the celiac lymph nodes, and this information may help guide management.
Magnetic Resonance Imaging (MRI)
MRI also has a role in imaging the pancreatic duct and cystic lesions of the pancreas but the choice of MRI versus CT as the primary imaging modality is largely institutiondependent. The authors’ institution favors CT, but recognizes that MRI can be useful in identifying pancreaticobiliary anatomy and pathology. With increasing numbers of pancreatic resections being done for intraductal papillary mucinous neoplasms, MRI has become more frequently used as a diagnostic and screening tool in some institutions. In addition, important information about the pancreatic duct can be noninvasively elucidated using MRCP. This is particularly important when assessing a distal bile duct stricture, or when planning a pancreatic drainage procedure in the setting of chronic pancreatitis.
Positron Emission Tomography (PET)
There is currently insufficient evidence to advocate for the regular use of PET scanning in the diagnosis and staging of pancreaticobiliary disease. As with CT scan, the sensitivity of PET is closely associated with the size of the lesion, making it susceptible to missing low-volume metastatic disease.
Patient Preparation
The sum total of risk that major abdominal surgery poses to the patient is a function of several factors, including not only medical comorbidities, but also surgeon experience and institutional capability. Multiple studies have shown a reproducible correlation between good outcomes and both institution volume and surgeon volume for complex operations such as pancreatic resections.
Medical Considerations
The goal of the preoperative medical evaluation is not only to achieve “medical clearance” or “cardiac clearance” but also to optimize the patient’s health and to minimize risk. The predominant nonsurgical contributor to postoperative morbidity and mortality is the cardiovascular system, and given that perioperative myocardial infarction has been associated with a mortality rate as high as 70%, preoperative cardiac evaluation is essential. The elective, nonemergent nature of most pancreaticobiliary procedures allows, and indeed demands, preoperative medical optimization. Major abdominal surgery is considered by the American College of Cardiology an “intermediate risk” procedure, which carries a 1% to 5% chance of myocardial infarction or death of cardiac etiology. Patient-specific factors contributing to this risk which should draw attention include recent myocardial infarction, unstable angina, symptomatic arrhythmias, severe heart block, worsening or new-onset heart failure, and advanced aortic or mitral valve stenosis. In any of these situations, it is recommended that patients have preoperative evaluation and intervention, if required, in order to define and to minimize risk.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree