Chapter 56 Pancreatic and periampullary tumors
Classification and pathologic features
Tumors of the Pancreas
Pancreatic neoplasms are classified based on the degree to which they recapitulate one of the cellular components of the pancreas (Hruban et al, 2007; Klimstra & Adsay, 2009; Klimstra et al, 2007b). To appreciate the various tumor types that occur in the pancreas, basic functional and histomorphologic characteristics of the elements that constitute this gland, both neuroendocrine and exocrine, must be recognized. The neuroendocrine component consists of the islets of Langerhans, small nests of up to a few hundred cells, scattered throughout the pancreas, usually forming round, well-delineated units amid the exocrine elements. Islet cells synthesize hormones (i.e., insulin, glucagon, and somatostatin) and store them in neurosecretory granules; under appropriate stimuli, these hormones are released into the surrounding capillary network. Pancreatic neoplasms that recapitulate the islet cells were previously referred to as islet cell tumors but are now called pancreatic neuroendocrine tumors (PanNETs; see Chapter 61).
The ductal component of the pancreas is responsible for most of the neoplasms (see Chapter 58A, Chapter 58B ), but it is perhaps the least sophisticated. Ducts are responsible for transporting the acinar secretions to the duodenum. The ductal system starts with the centroacinar cells, and through intralobar and interlobar ducts, the enzymatic secretions are carried to the main pancreatic duct and eventually to the duodenum through the ampulla of Vater. The larger ducts produce and secrete mucins for lubrication and protection, to aid the passage of acinar products, and to create the proper milieu for the enzymes during transportation. However, intracellular mucin is not normally detectable in cells of the interlobular or smaller ducts. Recapitulating the normal ducts, ductal neoplasms are characterized by gland, duct, or cyst formation with or without papillary structures. Production of variable amounts of mucin is also typical and ranges from focal intracellular to abundant intracystic or stromal. As in any organ of the body, supportive tissues (mesenchymal elements) and neighboring organs may also give rise to tumors in the pancreas, although these are very rare. This section reviews the general characteristics of pancreatic neoplasms.
Invasive Ductal Adenocarcinoma
Invasive ductal adenocarcinoma is the most common neoplasm of the pancreas. Its clinical characteristics are discussed in detail elsewhere in this text (see Chapter 58A, Chapter 58B ). Most are solid, ill-defined masses; conversely, most solid, ill-defined lesions in the pancreas also prove to be ductal adenocarcinoma. Ductal adenocarcinomas have a remarkable tendency for rapid dissemination and insidious infiltration. Typically, it spreads in the abdomen in a multinodular fashion (intraabdominal carcinomatosis) or is already widely metastatic by the time the primary tumor grows to 5 to 6 cm in size. This feature is so characteristic that a larger solitary pancreatic mass is unlikely to be ductal adenocarcinoma. Interestingly, however, despite the high frequency of distant metastases, some ductal carcinomas cause death of the patient as a result of predominantly local extension; such cases appear to be molecularly distinctive.
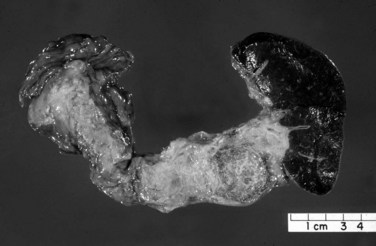
Macroscopically, ductal adenocarcinoma is a scirrhous (scar-forming) type of carcinoma; it is associated with abundant desmoplastic stroma, in which the neoplastic glands are widely scattered. This creates a challenge in the diagnosis of ductal adenocarcinoma, because often only a few cancer cells are present, if any, for evaluation in biopsy specimens. This is also a problem for cancer researchers, because most procured “tumor” specimens may in fact contain much more host tissue than carcinoma cells. Also, because ductal adenocarcinoma is typically very sclerotic, it can be difficult to distinguish from fibrosis of chronic pancreatitis, not only radiologically and intraoperatively but also for pathologists examining the gross specimen. The characteristics that distinguish ductal adenocarcinoma are its firm, gray, and gritty cut surface rather than the rubbery, milky white appearance of benign fibrotic lesions (Fig. 56.1).
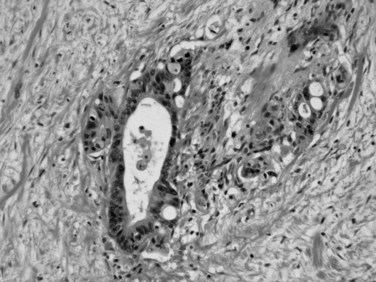
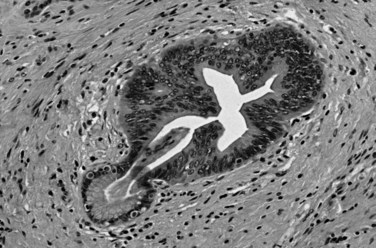
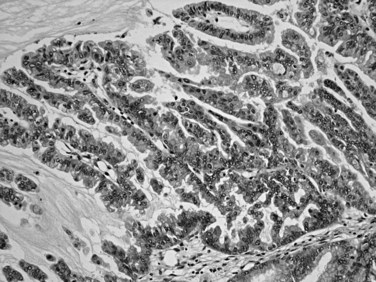
Microscopically, ductal adenocarcinoma is characterized by infiltrating tubular glands, often widely scattered, embedded in a desmoplastic stroma of variable cellularity (Fig. 56.2). The ducts are lined by cuboidal to columnar cells, with variable amounts of intracellular mucin and nuclear atypia (Klimstra & Adsay, 2009). The columnar cells may form cords or nests, or they may grow as individual cells. Perineural invasion is present in more than 60% of cases and is often identifiable in the retroperitoneal region or around the common bile duct, even away from the grossly evident tumor (Fig. 56.3). Neoadjuvant therapy seems to cause substantial alterations in the morphology of the tumor cells. Also, residual foci of previously treated ductal adenocarcinoma may be patchy and may require more careful examination. Recently, scoring systems have been devised in an attempt to evaluate the efficacy of chemotherapy; however, the relevance of these proposals requires further study.
The difficulty in distinguishing ductal adenocarcinoma from chronic pancreatitis (see Chapter 55A, Chapter 55B ) also applies to the microscopic diagnosis and is regarded to be one of the most difficult distinctions in diagnostic pathology (Adsay et al, 2004a). Chronic pancreatitis may be associated with epithelial atypia, both architectural and cytologic, in pancreatic tissue; conversely, ductal adenocarcinoma is notorious for its deceptively bland appearance. Features favoring a malignant diagnosis include abnormal location of glands (adjacent to muscular arteries, within the duodenal muscularis, adjacent to adipocytes in the peripancreatic tissue, or around nerves), architectural abnormalities in the shape of the glands (cribriforming, angulation, or incomplete gland formation), and nuclear abnormalities (variation in the shape and size of nuclei among the cells within an individual gland). A fourfold variation in nuclear volume between adjacent cells, otherwise known as the 4-to-1 rule, is helpful to recognize carcinoma. Diagnostic difficulty also extends to the differential diagnosis of ductal adenocarcinoma in metastatic sites, because ductal adenocarcinoma often retains its well-differentiated appearance and mimics benign or low-grade neoplasms of these sites. Common pitfalls include misinterpretation of metastatic ductal adenocarcinoma in the ovary as a primary borderline ovarian cystadenoma (Young, 2007); in the lung, as bronchioloalveolar carcinoma; and in the liver, as bile duct adenoma. In the last instance, the converse—misinterpretation of bile duct adenoma as metastatic ductal adenocarcinoma—seems to be more common.
No uniformly applied pathologic grading system exists for ductal adenocarcinoma. Schemes advocated by Western and Asian experts show major philosophical differences in principle and results. The current American Joint Commission on Cancer–endorsed tumor-node-metastasis (TNM) grading system (Edge et al, 2010) is similar to the grading of other adenocarcinomas of the gastrointestinal (GI) tract: well differentiated means 95% or more composed of glandular structures; moderately differentiated, 50% to 95% glandular in pattern, the remainder occurring as solid nests or individual cells; and poorly differentiated, with more than 50% solid nest and individual cells. The World Health Organization (WHO) has adopted the complex grading scheme proposed by Klöppel and colleagues, which is difficult to employ and not widely used in daily practice (Fukushima, 2010). Intratumoral heterogeneity seems to be an important problem in the grading of ductal adenocarcinoma, and for this reason, a simpler, more practical, and more clinically relevant grading scheme that accounts for this heterogeneity by scoring the patterns of infiltration has been proposed (Adsay et al, 2005).
The pathologic evaluation of a pancreatectomy specimen is important both for staging and in determining the adequacy of resection (Lüttges et al, 1998; see Chapter 58A, Chapter 58B ). Extrapancreatic extension and involvement of neighboring organs are documented in the pathology report, and determination of tumor size is important. As stated earlier, it is often difficult to appreciate the boundaries of ductal adenocarcinomas; careful macroscopic examination and close correlation with the microscopic findings are necessary to establish the correct size of the tumor. Microscopic foci of carcinoma are often present within the pancreas distant from the main tumor (Bandyopadhyay et al, 2009). Metastasis to lymph nodes is considered one of the most important predictors of outcome in resected ductal adenocarcinomas. Generally, at least 12 lymph nodes should be identified in a simple pancreatoduodenectomy specimen (Adsay et al, 2009). Most of these lymph nodes are embedded in the surfaces of the pancreas or in the groove between the pancreas and duodenum.
Proper identification of margins and their adequate sampling also are important components in the pathologic evaluation of a pancreatoduodenectomy specimen (Ferrone et al, 2008). For pancreatic ductal adenocarcinoma, the most important margin seems to be the retroperitoneal margin, also termed the uncinate, superior mesenteric vein, or vascular margin (Staley et al, 1996; Lüttges et al, 1998). The retroperitoneal margin at the posteroinferior aspect of the head of the pancreas can be distinguished by its nodular, granular, and irregular appearance, with the vascular bed of the superior mesenteric vessels appearing as a groove to its left. Studies have shown that the retroperitoneal margin should be sampled thoroughly, because this margin is often microscopically positive for grossly undetectable disease. Common bile duct, distal pancreatic (pancreatic ductal), and GI mucosal margins also should be evaluated.
As expected, ductal adenocarcinoma shows immunohistochemical evidence of ductal differentiation. Mucin-related glycoproteins and oncoproteins, including carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), B72.3, DUPAN2, MUC1, and MUC5AC are commonly detected in ductal adenocarcinoma (Basturk et al, 2010b; Hruban et al, 2007; Klimstra, 1998). Expression of these and the differential labeling for cytokeratins (CK7 positive, CK20 variable) sometimes may help distinguish ductal adenocarcinoma from other carcinomas, although no profile of immunohistochemical markers is specific for ductal adenocarcinoma. Scattered cells with neuroendocrine differentiation may be present, and acinar enzymes typically are not expressed.
Substantial developments have occurred in understanding the molecular carcinogenesis of ductal adenocarcinoma (see Chapter 8A). Mutation in codon 12 of the KRAS oncogene is found in more than 90% of ductal adenocarcinoma and seems to be an early event (Hruban & Adsay, 2009). Mutation of P16 or methylation of the promoter also is common (>80%) and represents the pathogenetic link with the familial atypical multiple mole–melanoma syndrome (Hruban et al, 2001a). Overexpression of TP53 (Barton et al, 1991; Hameed et al, 1994) and loss of SMAD4/DPC4 are detected in about half of cases (Tascilar et al, 2001). The latter appears to have a modest degree of specificity for pancreatic ductal adenocarcinoma. Immunohistochemical demonstration of these abnormalities may be useful in the diagnosis of carcinoma (Fig. 56.4). BRCA2 and Peutz-Jeghers gene mutations have been implicated in about 5% of ductal adenocarcinoma cases (Hruban & Adsay, 2009). Fanconi anemia gene alterations also have been identified (van der Heijden et al, 2003). Abnormalities in mismatch repair proteins and microsatellite instability are uncommon, although pancreatic ductal adenocarcinomas can occur as one of the less common manifestations of Lynch syndrome.
Pancreatic Intraepithelial Neoplasia (PanIN)
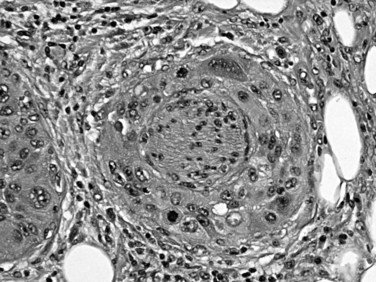
It has been speculated for decades that proliferative lesions in the ducts precede the development of invasive ductal carcinoma (Klöppel et al, 1980). These proliferations have been referred to by a plethora of terms, including hyperplasia and dysplasia, but these are now designated pancreatic intraepithelial neoplasia (Hruban et al, 2001b) in recognition of their neoplastic nature (Fig. 56.5). The spectrum of changes is classified in three grades (Hruban et al, 2001b). Replacement of the normal cuboidal, nonmucinous ductal epithelium with columnar cells that contain abundant apical mucin, but without architectural complexity (e.g., papilla formation) or cytologic atypia, is regarded as PanIN1A. Cytologically similar lesions that form papillae constitute PanIN1B. When a substantial pseudostratification of the cells and some degree of cytologic atypia are seen, the lesion is PanIN2. When irregular papillary architecture is present with tufting, cytologic atypia, necrosis, suprabasal mitoses, and loss of cell polarity, it is regarded as PanIN3.
A progressive accumulation of molecular alterations is reported from PanIN1 to invasive carcinoma (Hruban & Adsay, 2009). Some alterations, such as KRAS mutations, are early events; others, such as TP53 overexpression, occur at the more advanced end of this spectrum. Low-grade PanINs are very common incidental findings in the normal population (Andea et al, 2003); therefore they are generally believed to not to require any further clinical attention, if encountered in isolation or at resection margins. PanIN3, on the other hand, is seldom seen in the absence of an invasive carcinoma (Andea et al, 2003), and for this reason, if PanIN3 is encountered in a pancreas, the likelihood of pancreas cancer elsewhere in the gland is very high.
Other Invasive Carcinomas Related to Ductal Adenocarcinoma
Certain types of carcinomas are closely related to, and often seen in association with, ductal adenocarcinoma (Klimstra & Adsay, 2009). Undifferentiated carcinoma can be regarded as the least differentiated form of ductal adenocarcinoma, in which characteristic tubule formation is no longer evident or only focal. Such tumors are rare, and their demographics do not seem to differ from ordinary ductal adenocarcinoma, except that they may have even more aggressive behavior. Undifferentiated carcinomas include sarcomatoid (spindle cell) carcinoma, anaplastic giant cell carcinoma, and carcinosarcoma. Rarely, the sarcomatoid components of these tumors may show aberrant differentiation, including bone and cartilage formation.
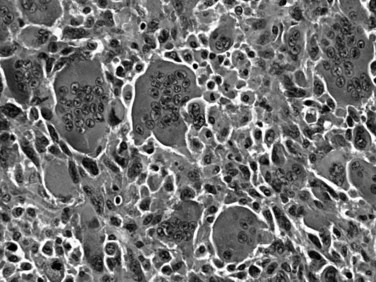
Also known as osteoclastic giant cell carcinoma, undifferentiated carcinoma with osteoclast-like giant cells is the name commonly used for a distinctive tumor type characterized by an abundance of such cells in the background of a sarcomatoid carcinoma (Fig. 56.6; Hoorens et al, 1998; Westra et al, 1998). Studies have shown that the osteoclastic giant cells are nonneoplastic histiocytic cells (Westra et al, 1998); the reasons for this chemotaxis are unknown. The true neoplastic cells in this tumor type are the sarcomatoid mononuclear cells. An adenocarcinoma component or, in some cases, high-grade PanIN or mucinous cystic neoplasm precursors may be present. Undifferentiated carcinomas with osteoclast-like giant cells often appear well demarcated and form a large solitary mass. If examined carefully, many such tumors appear to have substantial intraductal growth. These are clearly malignant neoplasms, most exhibiting an aggressive clinical course; however, some examples with minimal ductal adenocarcinoma components have a protracted clinical course.
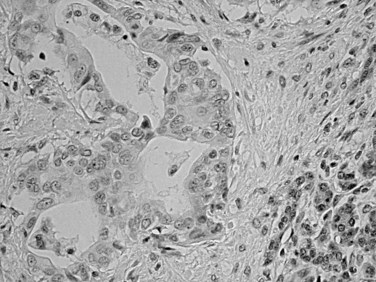
Squamous differentiation is seen in some conventional ductal adenocarcinomas (i.e., adenosquamous carcinomas; Fig. 56.7; Kardon et al, 2001), but rare pure examples of squamous cell carcinoma without any glandular components also may be seen. They may have variable degrees of keratinization. Squamous cell carcinoma and adenosquamous carcinoma of this region are highly aggressive tumors (Kardon et al, 2001) with a prognosis that is even worse then that of ordinary ductal adenocarcinoma.
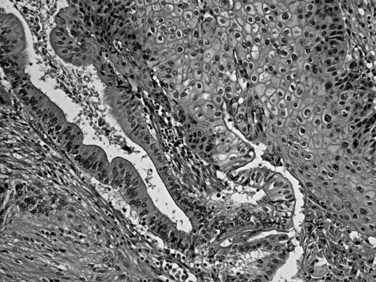
FIGURE 56.7 Adenosquamous carcinoma. Glandular differentiation and pavement-like squamous morphology are apparent.
Colloid carcinoma, pure mucinous or mucinous noncystic carcinoma (Adsay et al, 2001; Hruban et al, 2007; Seidel et al, 2002), is characterized by extensive stromal mucin deposition (Fig. 56.8). Those tumors composed almost exclusively of the colloid pattern—in which the mucin/epithelium ratio is very high, and most carcinoma cells are floating within the mucin (detached from the stroma)—have a different biology with an unusually protracted clinical course. Anecdotal evidence suggests that open biopsy of colloid carcinomas may contribute to dissemination, presumably owing to the adherent nature of the mucin. Colloid carcinomas also tend to be larger and better demarcated than ductal adenocarcinomas, and their molecular alterations seem to be somewhat unique. They usually are associated with intraductal papillary mucinous neoplasms (IPMNs) of the intestinal type (see Chapter 57) and also exhibit immunohistochemical evidence of intestinal differentiation (see later).
Medullary carcinomas, akin to those seen in the GI tract associated with microsatellite instability, also have been reported in the pancreas (Goggins et al, 1998; Wilentz et al, 2000). Syncytial nodules of large, poorly differentiated epithelioid cells with a pushing pattern of invasion characterize medullary carcinomas. In one study (Goggins et al, 1998), these tumors were found to have a more protracted clinical course, but further data are necessary to define the prognosis of these rare tumors.
Intraductal Neoplasms
Intraductal neoplasms constitute an increasingly encountered and important category of pancreatic tumors of ductal origin, characterized by intraductal polypoid/papillary nodules that are often associated with cystic dilatation of the ducts (Basturk et al, 2009). Intraductal neoplasms constitute a wide spectrum of morphologic variants, but they are all regarded to represent precursors to invasive carcinoma; that is, they may be associated with, or may progress to, invasive carcinoma. As preinvasive neoplasms, they are similar to PanINs; but in contrast to PanINs, which are incidentally detected microscopic forms of dysplasia, intraductal tumors form clinically detectable masses (defined as >1.0 cm), conceptually similar to adenomas of the GI tract. It is estimated that about 2% of invasive adenocarcinomas in the pancreas arise from these tumor types. The neoplasms that are included under this umbrella are intraductal papillary mucinous neoplasms and intraductal tubulopapillary neoplasms.
Intraductal Papillary Mucinous Neoplasms (See Chapter 57)
Intraductal papillary mucinous neoplasms (IPMNs) arise predominantly in the head of the gland and occur more commonly in elderly men (males ≥ females; mean age, 64 years; Basturk et al, 2009). The mucin produced by these tumors may exude from the ampulla of Vater, a finding that is virtually diagnostic of an IPMN. Radiographic findings of ductal dilatation with irregularities also are often diagnostic. A history of pancreatitis is noted in some patients. An adenoma-to-carcinoma progression is seen in IPMNs, which are correspondingly classified as having low-, intermediate- or high-grade dysplasia based on the greatest degree of atypia (Hruban et al, 2007; Adsay et al, 2010); invasive carcinomas that arise in IPMNs are recognized separately and are graded and staged like other ductal-type carcinomas. IPMNs that predominantly involve the secondary ducts (branch duct type; Tanaka et al, 2006; Schnelldorfer et al, 2008; Nagai et al, 2009; Koizumi et al, 2009; Tanno et al, 2008, 2010; Terris et al, 2000) tend to be small (<3 cm) and noncomplex, and they generally prove to have low- to intermediate-grade dysplasia, with simple, gastric foveolar-type epithelium by microscopic examination. IPMNs that mostly involve the major ducts (main duct type; Fig. 56.9) (Tanaka et al, 2006) more commonly harbor high-grade dysplasia or invasive carcinoma (Salvia et al, 2004).
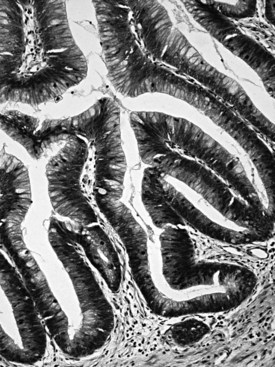
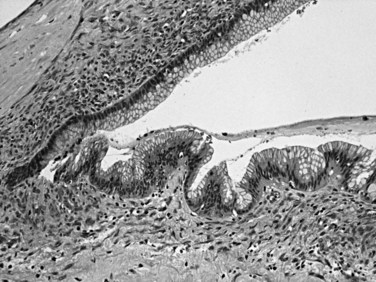
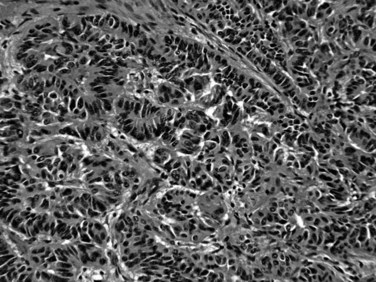
Distinctive pathologic subsets of IPMNs are emerging (Adsay et al, 2004b; Furukawa et al, 2005) with different histologic characteristics. In intestinal-type IPMNs, the papillary nodules are morphologically identical to colonic villous adenomas (Fig. 56.10), and the invasive carcinomas that develop in these tend to be of the relatively indolent colloid type (Adsay et al, 2001; Seidel et al, 2002). Intestinal-type IPMNs and colloid carcinomas typically express intestinal differentiation markers (MUC2 and CDX2) not found in ductal adenocarcinomas or in the nonintestinal subtypes of IPMNs discussed below, indicating that they represent a distinct “intestinal pathway” of carcinogenesis in the pancreas (Adsay et al, 2004b). Other IPMNs have more complex papillae that resemble papillary carcinomas of the biliary tree (Fig. 56.11). These pancreatobiliary-type IPMNs tend to be associated with tubular-type invasive carcinoma (conventional ductal adenocarcinoma) and appear to have more aggressive behavior (Sadakari et al, 2010). What is also known as intraductal oncocytic papillary neoplasm (Adsay et al, 1996) is now regarded as a distinct subtype of IPMN, characterized not only by the oncocytic nature of the cells but also by the complexity of the papillary nodules, which have an arborizing pattern. Oncocytic IPMNs are often large and very floridly proliferative; however, the carcinomas arising from them tend to be small, the incidence of metastasis is low, and the overall prognosis appears to be favorable. They also have different molecular changes from other IPMNs, with a much lower incidence of KRAS mutation and frequent expression of MUC6, suggesting a pyloropancreatic lineage distinct from the intestinal-type IPMNs (Basturk et al, 2010b).
IPMNs can be multifocal in up to 40% of patients. They seem not only to be precursors but also “markers” of invasive adenocarcinoma (i.e., in some cases, invasive carcinoma is detected elsewhere in the gland, away from the IPMN; Fujii et al, 1996; Yamaguchi et al, 2002). Invasive carcinoma of either the colloid or tubular type (Adsay et al, 2004b) is present in more than one third of resected IPMNs. Colloid-type invasion occurs almost exclusively in intestinal-type IPMNs. It is generally believed that IPMNs proven devoid of high-grade dysplasia or invasive carcinoma by thorough histologic examination are innocuous, curable neoplasms. Patients with high-grade dysplasia may on occasion experience recurrences and metastases (White et al, 2007), presumably the result of undetected foci of invasive carcinoma; invasive carcinoma has a malignant clinical course, albeit often significantly more protracted than that of ordinary ductal adenocarcinoma. It should be kept in mind that most patients with IPMNs are older, often with comorbid diseases—including another malignant neoplasm in one third of patients (Eguchi et al, 2006; Sugiyama & Atomi, 1999; Adsay et al, 2002a; Adsay, 2008). The prognosis and management of IPMNs are discussed in detail elsewhere in this book (see Chapter 58A, Chapter 58B ).
Intraductal Tubulopapillary Neoplasms
Intraductal tubulopapillary neoplasm (Adsay et al, 2004b; Tajiri et al, 2005; Yamaguchi et al, 2009; Klimstra et al, 2007c) is a recently recognized category of mass-forming (>1.0 cm) intraductal neoplasm that is fairly similar to an IPMN, from which it is distinguished microscopically by its mucin-poor nature and distinctive tubular architecture. First reported by Tajiri and colleagues (2005) under the heading of intraductal tubular adenocarcinoma, the entity is now being designated intraductal tubulopapillary neoplasm in the new WHO classification. It is a rare tumor seen at an average age of 53 years, and it presents with nonspecific symptoms. The clinical findings are often indistinguishable from those of IPMNs. Most cases have cystic ducts filled with tumor nodules. Intraductal tubulopapillary neoplasm occurs predominantly in the head of the pancreas but may involve any part. It is often large (mean, 7 cm; range, ≤15 cm).
It should be noted that a variety of pancreatic neoplasms can show prominent intraductal growth, including acinar cell carcinomas (Basturk et al, 2007; Ban et al, 2010), pancreatic neuroendocrine tumors, osteoclastic giant-cell carcinomas (Basturk et al, 2010c), and even metastatic tumors; thus, they fall into the differential diagnosis of these intraductal neoplasms.
Mucinous Cystic Neoplasms (See Chapter 57)
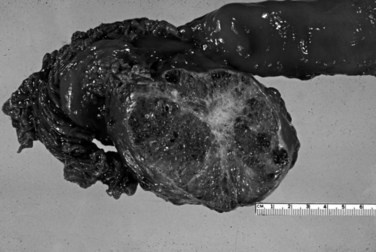
Mucinous cystic neoplasms (MCNs) are seen almost exclusively in perimenopausal women (>95% female; mean age, 48 years; Tanaka et al, 2006; Thompson et al, 1999; Zamboni et al, 1999). One of the characteristic features, which essentially has become a requirement for the diagnosis, is an ovarian-type subepithelial stroma that is not only histologically similar to the ovarian cortex but that also expresses progesterone and estrogen receptors by immunohistochemistry. This distinctive mesenchyme helps distinguish MCNs from similar neoplasms, especially IPMNs (see above). MCNs typically form a thick-walled multilocular cyst in the body or tail of the pancreas (Fig. 56.12). Some examples may mimic pseudocysts, although the surrounding pancreatic tissue typically does not show evidence of chronic pancreatitis. In most cases, cysts do not communicate with the ductal system. The cyst fluid is rich in mucin-related glycoproteins and oncoproteins, such as CEA (Pitman et al, 2010; Deshpande et al, 2006), which may help distinguish these tumors from other cystic lesions. The cysts are lined by mucinous epithelium, which may exhibit varying degrees of cytologic and architectural atypia (Fig. 56.13), graded as low-, intermediate-, or high-grade dysplasia based on the most atypical region. Invasive carcinoma typically occurs in larger and more complex cases that show florid papillary nodules in the cysts.
Invasive carcinomas are found in 10% to 15% of MCNs (Pitman et al, 2010; Zamboni et al, 1999; Reddy et al, 2004). The carcinoma may be limited to microscopic foci within the septa of the cysts, or it may invade out of the cyst into the peripancreatic tissues. Invasive carcinomas resemble ductal adenocarcinoma histologically, including the occasional occurrence of an uncommon histologic variant such as sarcomatoid or giant-cell carcinoma. Although some studies have questioned the utility of grading MCNs, it has become clear recently that if these tumors are examined thoroughly, and the presence of invasive carcinoma is ruled out by rigorous pathologic examination, patients typically are cured by complete excision (Zamboni et al, 1999; Sakorafas & Sarr, 2005; Hruban & Zamboni, 2009). Because all MCNs have the potential to harbor invasive carcinoma, most authors agree that these tumors should be completely resected.
Serous Cystic Tumors (See Chapter 57)
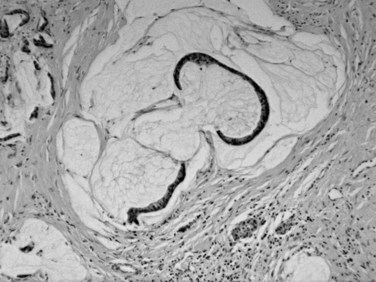
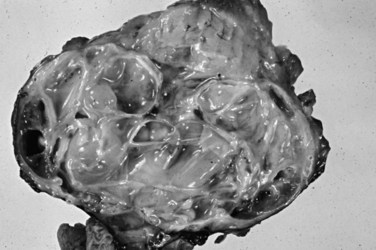
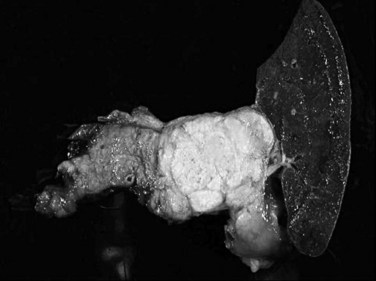
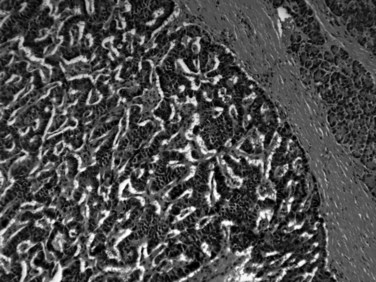
Serous cystadenomas (Bassi et al, 2003; Compton, 2000) form relatively large masses (mean size, 9 cm, some 25 cm) that tend to be well demarcated, predominantly in elderly women (mean age, 63 years; female/male ratio, 3 : 1). Typically, serous cystadenomas are composed of thousands of small cysts, each measuring only millimeters and creating the diagnostic macroscopic appearance of a spongelike configuration, hence the synonym microcystic adenoma (Fig. 56.14). Rare macrocystic and solid examples of serous adenomas also have been described. Microcystic serous cystadenomas often have a central stellate scar. The defining microscopic feature is a simple, nonmucinous, cuboidal epithelium that contains intracytoplasmic glycogen, resulting in characteristic clear cytoplasm (Fig. 56.15). Serous cystic tumors are one of the few ductal neoplasms of the pancreas that do not produce mucin, possibly reflecting a recapitulation of the centroacinar cells that are nonmucinous. Along the same lines, the cyst contents are devoid of the mucin-related glycoproteins and oncoproteins that typically are found in mucinous pancreatic tumors, a feature that may help in the preoperative diagnosis (Brugge et al, 2004). Microscopically, these lesions are similar to the cysts seen in von Hippel–Lindau (VHL) disease, and some serous cystadenomas do show VHL gene alterations. Serous cystadenomas often are reported to coexist or “collide” with other pancreatic neoplasms and with congenital pathologic conditions (Compton, 2000).
Malignant serous tumors (serous cystadenocarcinomas) (Matsumoto et al, 2005; Shintaku et al, 2005; Strobel et al, 2003) are exceedingly rare. Only the presence of recurrence, metastases, or angioinvasive growth separates these tumors from their benign counterparts (i.e., the cytologic appearance is entirely bland). For practical purposes, serous cystadenomas limited to the pancreas are regarded as uniformly benign.
Pancreatic Neuroendocrine Tumors (See Chapter 61)
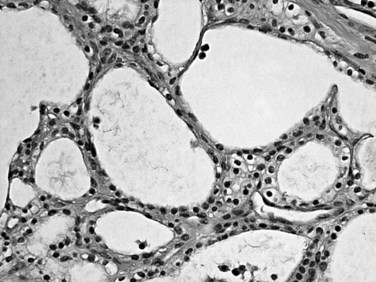
Most neuroendocrine-type tumors of the pancreas are well-differentiated, low- to intermediate-grade neuroendocrine tumors, previously referred to as islet cell tumors and now designated as pancreatic neuroendocrine tumors (PanNETs) by the World Health Organization (WHO) (Klimstra et al, 2010a; Solcia et al, 1991), with low-grade malignant behavior (Hochwald et al, 2002). Grossly, PanNETs are usually solid, circumscribed, and fleshy tumors, although multinodular and sclerotic examples occur (Fig. 56.16). Rarely, cystic degeneration may be seen (Adsay & Klimstra, 2000; Deshpande & Lauwers, 2007), with a central unilocular cyst lined by a cuff of viable tumor; this occurs more often in the setting of multiple endocrine neoplasia (MEN) syndrome type I. PanNETs recapitulate the morphologic features of islet cells by forming nested, gyriform, trabecular, or rarely acinar or glandular patterns (Fig. 56.17). The cells have characteristic neuroendocrine features, including round, monotonous nuclei with a coarsely stippled chromatin pattern and moderate amounts of cytoplasm (Fig. 56.18).
Almost half of PanNETs are clinically functional, and patients come to medical attention with signs or symptoms of inappropriate production and serologic activity of one or more hormones. These functional PanNETs are named based on the hormonal syndrome: insulinoma, glucagonoma, gastrinoma, somatostatinoma, and VIPoma (vasoactive intestinal peptide tumor) for example. The correlation of serum hormone levels and expression of the corresponding hormone in the tumor itself by immunohistochemistry is often imperfect. Nonfunctional PanNETs (Hochwald, et al, 2001; Klimstra & Adsay, 2009; La Rosa et al, 1996) constitute an ever-enlarging proportion of PanNETs because they are being detected more commonly as incidental findings on abdominal imaging studies. Most insulinomas follow a benign clinical course, likely because insulinomas typically are highly symptomatic, even when they are small, which leads to their early detection. Glucagonomas, on the other hand, tend to be large at diagnosis and have a more aggressive course. PanNETs associated with MEN type I or other syndromes tend to be multifocal and less aggressive (Anlauf et al, 2006, 2007; Klimstra & Adsay, 2009; Perigny et al, 2009). In addition to grossly evident and usually functional PanNETs, patients with MEN type I have numerous neuroendocrine microadenomas, defined as PanNETs (<0.5 cm). Most sporadically occurring functional and nonfunctional PanNETs are clinically low-grade malignancies. More than half of patients have recurrence or metastasis after resection, and many patients come to attention only after the development of metastatic disease. Nonetheless, there may be a relatively protracted clinical course even in patients with metastatic disease. It has been difficult, as in neuroendocrine tumors of other organs, to determine which PanNETs are more likely to metastasize and which metastatic cases are likely to progress most rapidly (Pelosi et al, 1996; White et al, 1994). Findings associated with more aggressive behavior include size greater than 3 cm, a functional PanNET other than insulinoma, extrapancreatic or vascular invasion, high mitotic activity, high proliferation index (based on immunohistochemical staining for Ki-67) (Jamali & Chetty, 2008; Rindi et al, 2007; Chatzipantelis et al, 2009), CK19 expression (La Rosa et al, 2007), and c-KIT expression (Zhang et al, 2009); however, some PanNETs lacking all of these features still may metastasize.
The 2004 WHO classification (Heitz et al, 2004) separated well-differentiated pancreatic neuroendocrine tumors, those lacking metastases or gross local invasion, from well-differentiated pancreatic neuroendocrine carcinomas, with metastases or gross local invasion; they further predicted “benign behavior” or “uncertain behavior” in the former group based on size less than 2 cm, mitotic rate below 2/10 high-power fields (HPFs), Ki-67 labeling index below 2%, and absence of vascular or perineural invasion. However, studies have demonstrated that the patients who fall in the uncertain behavior group still experience recurrence and metastasis in long-term follow-up in up to 40% of the cases (Schmitt et al, 2007); therefore it is clear that even this group represents a low-grade malignancy.
Another more recent proposal (Ferrone et al, 2007) divides PanNETs into low-grade and intermediate-grade groups based on the mitotic rate (2 mitoses per 50 HPFs) and necrosis. The two groups exhibit a highly significant difference in prognosis, although no subset specifically is regarded to be utterly benign. Recently, a multidisciplinary group of international experts have proposed a set of parameters to be included in pathology reports (Klimstra et al, 2010a). It was emphasized that PanNETs ought to be evaluated by the approach used for any other malignancy, and accordingly, the grade and stage should be reported separately (Klimstra et al, 2010b; Klöppel et al, 2010a). For grading, in 2010 the WHO adopted the system originally devised and tested by the European Neuroendocrine Tumor Society (ENETS), which grades PanNETs based on the mitotic count and Ki-67 labeling index. Grade 1 if the mitotic rate is less than 2/10 HPFs and the Ki-67 index is below 3%; grade 2 if the mitotic rate is 2 to 20/10 HPFs or the Ki-67 index is 3% to 20%; and grade 3 if either is above 20. For staging, the AJCC has adapted the TNM-based staging system used for adenocarcinomas, but the ENETS system is somewhat different (Klöppel et al, 2007).
On the other end of the spectrum of neuroendocrine neoplasia, very rarely, poorly differentiated neuroendocrine carcinoma, related to small cell carcinoma, may be encountered in the pancreas (Tranchida et al, 2000). Some are composed of large cells with more abundant cytoplasm (high-grade neuroendocrine carcinomas of a “large cell” type). According to WHO, high-grade neuroendocrine carcinomas are classified as grade 3 PanNETs, although the true nature of such tumors is difficult to characterize because of their rarity, and they appear to be a distinct tumor type with morphology and behavior usually very similar to that of high-grade neuroendocrine carcinomas of the lung rather than being advanced forms of well-differentiated PanNETs. In fact, especially when the small cell morphology is present, poorly differentiated neuroendocrine carcinomas must be distinguished from metastases to the pancreas from a pulmonary primary.