Mark S. Litwin, MD, MPH
Access to Care
Access to care includes the “actual use of personal health services and everything that facilitates or impedes their use. It is the link between health services systems and the populations they serve” (Andersen and Davidson, 2001). Andersen presented a behavioral model for considering health care access in which three categories of factors determine how and whether individuals use medical services. These include predisposing factors (e.g., health beliefs, attitudes); enabling factors (e.g., health insurance, geographic proximity); and need factors (e.g., the presence of symptoms or diseases) (Aday and Andersen, 1981; Andersen, 1995). Barriers to health care access often result from financial determinants such as the lack of health insurance or adequate income, logistic challenges in coordinating child care, public transportation, and work schedules; clinic waiting times; and difficulties with the geographic proximity of clinics and hospitals (Griffiths et al, 2004).
Adequate access requires more than a guarantee of payment for services; even with generous benefits, individuals must navigate nonfinancial barriers. Among these are minority status (Mandelblatt et al, 1999; Eisenberg and Power, 2000; Institute of Medicine, 2003; Lurie and Dubowitz, 2007); gender (Saigal et al, 2008); environmental factors (Davidson et al, 2004); health behaviors (Call et al, 2006); acculturation, language, and citizenship (De Alba et al, 2005; Echeverria and Carrasquillo, 2006; Flores, 2006); provider proximity (Schroen et al, 2005); available safety-net services (Call et al, 2006); and the absence of a usual source of care (Sox et al, 1998; Petterson et al, 2009).
Barriers to access may also result from more subtle factors such as fear of the health care system, ethnic disparities, cultural norms, embarrassment, perceived health status (Fitzpatrick et al, 1998), lack of self-efficacy, knowledge (Deibert et al, 2007), or special social circumstances. Even in health care systems that are considered to provide “equal access,” such as the U.S. Veterans Affairs hospitals, different patient groups use outpatient and inpatient care at markedly different rates, leading to variations in outcomes. Differences in access to care have been cited as a fundamental reason for disparities in health status among various populations (Kelley et al, 2005a, 2005b).
In this context, differential access to care has been proposed as an important determinant of racial/ethnic disparities in prostate cancer screening, treatment, morbidity, and mortality (Mandelblatt et al, 1999; Hoffman et al, 2001; Underwood et al, 2004; Talcott et al, 2007). This is supported by empiric data demonstrating that African-American men with prostate cancer are more likely than whites to be uninsured; to receive their usual medical care in a public clinic or emergency department; and to see different clinicians on subsequent prostate cancer–related visits (Talcott et al, 2007). Likewise, discrepancies in accessibility and continuity of medical care may explain the lower use and awareness of prostate specific antigen (PSA) testing among Hispanic men in the United States (Spencer et al, 2006; McFall, 2007); it remains unknown whether access or continuity issues mediate the greater dissatisfaction with prostate cancer treatment decisions among Hispanic men (Hoffman et al, 2003).
Access to health care reflects not only the potential for entry into the health care system, but also the actual consumption of services. In one public-assistance program for low-income, uninsured men with prostate cancer, special attention to overcoming the financial and nonfinancial barriers has eliminated racial/ethnic disparities in health services utilization (Miller et al, 2008a). Nonetheless, because men from historically disadvantaged groups often do not access adequate and timely care, they continue to suffer a disproportionate burden (Miller et al, 2009b).
Costs of Care
Urologists have long been attentive to issues of cost in managing health care. In his autobiography, Hugh Hampton Young, the father of American urology, described a patient on whom he agreed to perform a prostatectomy in the 1920s for a fixed fee of $500 with a promise of only 3 weeks in the hospital. Because the patient suffered complications and remained hospitalized for much longer than planned, Young had to spend his entire professional fee plus an additional $350 to pay off the hospital bill (Young, 1940).
Health policy decisions today are based not only on biomedical research but also on sound evaluations of health care costs. The introduction of oral erectogenics, beginning in the late 1990s, provides a perfect illustration of the tension between therapeutic advances and economic forces. The scientific discoveries that led to the advent of sildenafil and similar agents (Rajfer et al, 1992) were rewarded with a Nobel Prize, yet insurers initially fervently resisted paying for them (Lee, 1999). This led to potent controversy, generated numerous studies, and even raised constitutional law questions (Keith, 2000; Smith and Roberts, 2000; Connolly, 2001). As a surgical subspecialty with large numbers of Medicare patients, many of whom suffer waning turgor, urology is a discipline in which cost-saving maneuvers may have tremendous financial impact.
Terms and Methods of Analysis
In its purest form, research on health care costs involves counting the amount of money that is expended on facilities, equipment, supplies, and personnel during the provision of medical care. But many costs are hidden. A more extensive approach would also include the opportunity cost of the time patients spend receiving care. For example, when estimating the cost of an interval cystoscopy after resection of a bladder tumor, a thorough assessment would include not only the cost of overhead, supplies, lidocaine jelly, urine cytology, and professional fees but also the cost to the patient in lost wages—or to his or her employer in lost productivity—during the time away from work for the procedure, travel, or any complications. This component is not insignificant. Employee absence from work has been estimated to cost U.S. businesses tens of millions of dollars annually (Walsh et al, 1989; Luz and Green, 1997; Stewart et al, 2003).
To avoid the inherent problems in calculating costs and charges, some researchers instead measure resource utilization in terms of duration, frequency, and intensity of services (Munoz et al, 1988b). One of the most commonly reported units of comparative analysis is length of stay (LOS). After 1983, when the prospective payment system was instituted to reimburse hospitals a predetermined amount of money on the basis of the diagnosis-related group (DRG) into which each Medicare inpatient is classified (Munoz et al, 1988a; Kahn et al, 1990; Munoz et al, 1990a, 1990b, 1990c), attention to LOS as an outcome variable in cost analyses greatly increased. In the United States, inpatient LOSs are now believed to have reached their floor, and effort has shifted from this measure.
Cost-effectiveness analysis is another popular technique used to evaluate new or established medical therapies. A cost-effectiveness analysis is performed by developing a probability model of the possible medical outcomes of different interventions (or a nonintervention such as watchful waiting for benign prostatic hyperplasia) (Saigal et al, 2007), identifying the expenses associated with each outcome, and comparing the results, typically reported as cost per year of life saved (Shepard and Thompson, 1979; Henriksson and Edhag, 1987; Chandhoke and DeAntoni, 1998; Manca et al, 2003). Years of life saved, or life-years (LYs), are calculated for a population, not for individuals. Ten LYs might represent 2 patients, each of whom survives for 5 additional years, or 120 patients, each of whom survives for 1 additional month (Smith et al, 1993). LYs are usually adjusted to account for different health states that may result from various treatments. These are called quality-adjusted life-years (QALYs). For example, when comparing two treatments for localized prostate cancer, if both options are determined to cost $10,000 per year of life saved, then the two may seem equivalent. However, if one treatment yields years that are compromised by bothersome sexual dysfunction, while the other yields years that are free of such problems, then the difference in quality of the years saved must be factored into the equation. Analyses that rely on QALYs are facilitated by the estimation of patient utilities, or preferences, for various health states (Nease and Owens, 1994; Albertsen et al, 1998; Saigal et al, 2001; Saigal et al, 2002). If patients appraise an impotent year as being worth less than a potent year, then quality adjustments may make the first treatment more expensive per QALY saved. Cost-benefit analysis differs by including not only the costs but also the equivalent monetary value of any benefits garnered during the extra years of life. Often, this refers to wages earned or income accrued during that time. In more sophisticated analyses, future income and expenses related to a particular health state are discounted to present value by incorporating projected interest and inflation rates over time.
Cost studies may be undertaken as descriptive analyses that chronicle the economic burden of one disease in a group of patients, such as the cost of care for interstitial cystitis in a managed care plan (Clemens et al, 2008). They may examine the financial impact on the general population such as national annual expenditures for urolithiasis (Lotan, 2009). They may also track cumulative costs over time, such as for different prostate cancer treatments (Wilson et al, 2007). Alternatively, they may present economic models comparing different approaches to managing an illness such as whether it is more costly to correct cryptorchidism in infants or older boys (Hsieh et al, 2009), or to manage small renal tumors with laparoscopic or percutaneous cryoablation (Badwan et al, 2008).
Patterns of Care
Much of the current research into the cost of treating patients is predicated on the observation that patterns of care differ substantially for certain conditions. Physician demographics and patient age have been found to affect practice patterns (Bennett et al, 1991; O’Leary et al, 2000; Bird et al, 2003; Joudi et al, 2003; Fallon et al, 2005). The availability of evidence does not always predict the diffusion of new technologies (Michel, 2006), especially in urology. For example, robotic prostatectomy has seen nearly ubiquitous adoption, largely driven by overwhelming commercial and market forces, despite the absence of evidence showing superiority (Hu et al, 2006, 2009). Conversely, some advances with strong evidence-based advantages such as partial or laparoscopic nephrectomy for small renal masses or neobladder reconstruction following cystectomy, have diffused much more slowly (Gore et al, 2006; Miller et al, 2008b).
Despite the relative scarcity of cost analyses in urology, significant variations in length of stay and charges have been reported for many nonurologic medical and surgical hospitalizations (Fisher et al, 2003a, 2003b; Weinstein et al, 2004; Wennberg, 2004). Cost savings can be achieved by identifying variations in urologists’ practice habits and targeting them for reduction in intensity and duration (Sage et al, 1988; Kramolowsky et al, 1995a, 1995b). These changes in urologic practice have been shown to result in equivalent medical outcomes (Cleary et al, 1991), despite the finding that patients historically have tended to dislike shorter postoperative hospital stays (Litwin et al, 1997; Durieux et al, 2004). Overall, studies have been mixed. Some have described the successful use of cost-control mechanisms in urology (Cuckow, 1992a, 1992b), whereas others have failed to show sustained results (Forrest et al, 1981).
Analysis of both medical and surgical DRGs indicates that consistent patterns of resource utilization are identifiable during the course of hospitalizations, suggesting that elements of utilization may be managed more effectively to yield cost savings (Carter and Melnick, 1990). This is particularly true in treatments for genitourinary conditions such as hematuria, incontinence, or prostatitis (Taylor et al, 2008), many of which may be standardized. Urology is a fertile area for economic analysis because so little is known about the cost-effectiveness of screening and treatment interventions for even the most common genitourinary conditions (Perlman et al, 1996; Grossfeld and Carroll, 1998; Benoit et al, 2001; Mor et al, 2001; Shaw, 2004).
The advent of managed care has greatly assisted the study of practice patterns and medical outcomes. Most researchers agree that health maintenance organizations (HMOs) improve access to medical services and cost efficiency of care without compromising quality (Holtgrewe, 1998a, 1998b; Haffer and Bowen, 2004; Roetzheim et al, 2008). However, contrary evidence suggests that cancer patients may wait longer for treatment in HMOs than in fee-for-service plans (Greenwald, 1987). Among men newly diagnosed with prostate cancer, those cared for in HMOs are almost 1.5 times more likely to receive radiation than surgery for clinically localized tumors. This appears consistent with the general HMO emphasis on outpatient care. In this study, HMO patients had a lower overall mortality rate than did non-HMO patients. Interestingly, low-income men treated in HMOs enjoyed survival times that were significantly greater than for middle- or high-income men in HMOs or fee-for-service plans (Greenwald and Henke, 1992).
The National Institute of Diabetes and Digestive and Kidney Diseases funds the Urologic Diseases in America project (www.udaonline.net), which seeks to define the burden of urologic disease on the American public by quantifying trends in resource utilization, practice patterns, costs, outcomes, and epidemiology across the spectrum of urologic conditions (Litwin et al, 2005; Miller et al, 2009a). Documenting these trends has broad implications for quality of health care, access to care, and the equitable allocation of scarce resources, both in terms of medical services and research budgets. Among the most financially burdensome urologic conditions are urolithiasis (Lotan, 2009), urinary tract infection (Griebling, 2005a, 2005b), urinary incontinence (Anger et al, 2006), and benign prostatic hyperplasia (Nickel, 2006a, 2006b). Urologic diseases exert a substantial impact on resource utilization within the U.S. Veterans Affairs health system (Anger et al, 2008).
Case Mix
When studying patterns of care or medical costs, it is critical to adjust for case mix. Case mix refers to the severity of illness and degree of comorbidity in a group of patients (Iezzoni, 1996, 1997). These patient characteristics may influence treatment outcomes. For example, because they typically have a greater burden of comorbidity, older patients are more likely to experience complications after surgery. This comorbidity must be accounted for in evaluations of clinical outcomes (Glance et al, 2008). If this fact is not considered when comparing surgical complication rates across hospitals, evaluators may erroneously conclude that a hospital with an older patient population is providing poorer-quality care. To use outcomes to measure quality of care, we need to adjust for these other factors including baseline patient characteristics and intervening treatments. This adjustment (referred to as case-mix adjustment or risk adjustment) can be extremely complex, and the selection of factors must be carried out carefully so that outcomes can be interpreted accurately. For a variety of reasons, sicker patients cost more, and it is important to control for this difference in comparative analyses. When examining the factors that lead to higher hospital charges for more ill patients, two forces must be considered: duration and intensity of care. Duration is usually quantified as inpatient LOS, whereas intensity of care may be assessed as numbers of services or charges per day. Patients with greater comorbidity may remain hospitalized longer even if they do not receive more intense care during their stays. Duration and not intensity appears to be the primary force driving up hospital charges for sicker urology patients (Litwin et al, 1993).
Typically, comorbidity is quantified by extracting diagnoses from claims data (Klabunde et al, 2002); however, some comorbidity measures rely on patient self-report. Various comorbidity measures (Kaplan and Feinstein, 1974; Charlson et al, 1987; Greenfield et al, 1993, 1995; Crabtree et al, 2000; Di Gangi et al, 2003) have been used by researchers to adjust for case mix and predict mortality from competing causes in clinical studies. Although all perform reasonably well in the research setting, most are based on diagnoses from retrospective chart review and difficult to apply clinically (Albertsen et al, 1996).
The impact of case mix on urologic disease outcomes has been studied most broadly in men treated for prostate cancer. The presence and severity of cardiovascular disease negatively affect outcomes in men treated with radiation or surgery (van de Poll-Franse et al, 2008a, 2008b). Higher body mass index is associated with poorer outcomes after robotic prostatectomy. In obese men, surgical times are more than an hour longer, blood loss is higher, and positive margin rates are higher (Herman et al, 2007). Obese men undergoing open radical prostatectomy have more aggressive tumors, more positive margins, and higher rates of biochemical progression than nonobese men (Freedland et al, 2008), although their long-term survival is unaffected (Siddiqui et al, 2006). Comorbidity is more important than age in predicting perioperative mortality rates after radical prostatectomy (Alibhai et al, 2005, 2006; Daskivich et al, 2010a).
The available general comorbidity indices do not appear to be equally effective at adjusting for case mix in men with prostate cancer (Alibhai et al, 2008). The optimal comorbidity index for prostate cancer should be disease specific and use empirically derived weights for component conditions (Klabunde et al, 2007). The Total Illness Burden Index for Prostate Cancer (TIBI-CaP) is a patient-reported measure of comorbidity, which identifies patients at high risk for non–prostate cancer mortality. It is a robust predictor of both mortality and future quality of life. Men in the highest risk category are 13 times more likely to die of causes other than prostate cancer over a 3.5-year period than the men who had the lowest scores (Litwin et al, 2007; Daskivich et al, 2010b).
Comorbidities such as obesity also affect the incidence and severity of benign urologic conditions (e.g., urinary incontinence, chronic prostatitis/chronic pelvic pain syndrome, benign prostatic hyperplasia/lower urinary tract symptoms, erectile dysfunction) (Pontari et al, 2005; Bhojani et al, 2008a, 2008b; Subak et al, 2009). The interrelationships between erectile dysfunction, obesity, cardiovascular disease, and metabolic syndrome are complex (Bener et al, 2008; Yassin et al, 2008).
Cost Savings in Urology
Physicians have been largely successful at attenuating the increases in hospital costs by eliminating unnecessary hospital days, but maximal savings may have been reaped from this strategy (Bodenheimer, 2005). Nevertheless, continued tailoring of the intensity and duration of service to patient burden of illness may still be shown to reduce costs. Urology has played a central role in the ongoing struggle to balance the competing priorities of minimizing costs and maximizing quality in health care (Loughlin, 2003). One recent government strategy adopted in pursuit of this goal is Medicare’s pay-for-performance initiative, in which providers are differentially reimbursed on the basis of their adherence to various condition-specific quality indicators (Corrigan and Ryan, 2004), many of which also result in cost savings.
Quality of Care
Quality of care research evaluates “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge” (Lohr 1990; Lohr et al, 1992; Institute of Medicine, 2001). Several initiatives are under way to collect and disseminate performance information about medical care. For example, the National Committee on Quality Assurance (www.ncqa.org) provides information to health care purchasers about the comparative performance of health plans in the United States. The Joint Commission (www.jointcommission.org) applies outcomes-based quality measures to accredit hospitals. Hundreds of performance indicators have been collected in its National Library of Healthcare Indicators. The Foundation for Accountability (www.facct.org), a consumer organization, “creates tools that help people understand and use quality information, develops consumer-focused quality measures, supports efforts to gather and provide quality information, and encourages health policy to empower and inform consumers.”
The conceptual framework for the measurement of quality of care in medicine was established 45 years ago by Donabedian (Donabedian, 1966). In this model, quality of care measures are categorized into three domains—structure, process, and outcome. Structure of care includes the equipment, resources, and provider experience necessary to provide care. Examples include volume of cases and board certification of providers. Process of care refers to technical and interpersonal elements of care that transpire between doctor and patient such as the extent of the history and physical examination, documentation of the workup, and ordering of diagnostic and laboratory tests. Process measures are often considered to be the best measure of quality (Brook et al, 1996; Brook et al, 2000). Outcomes of care include survival rates, complications, and patient-reported outcomes (PROs) such as health-related quality of life. As underscored in his exposition on quality of care, Blumenthal argues that the most important new development in our current understanding of medical outcomes was the recognition that it is patients who define which outcomes are most important and whether or not they have been achieved (Blumenthal, 1996a, 1996b; Blumenthal and Epstein, 1996). Hence we have seen the advent of patient-reported outcomes as primary end points in clinical trials and the primary focus of much attention in evidence-based medicine. In 2006 the U.S. Food and Drug Administration formally issued guidance for the development of PROs to be used in its assessment of new drugs and devices (www.fda.gov) (Sloan et al, 2007).
Leading the national effort in the United States to establish an infrastructure for improving quality of care, the federal Agency for Healthcare Quality and Research (AHRQ) has published a series of quality indicators (www.qualityindicators.ahrq.gov). On the basis of extensive reviews of available evidence, AHRQ developed four categories of quality indicators (prevention, patient safety, inpatient, and pediatric), which are now being used to modulate hospital and physician reimbursement. In this model, quality of care is assumed to be directly related to adherence to these published quality indicators.
One substrate for the study of quality of care in urology is prostate cancer; it is prevalent, costly, and associated with great clinical uncertainty (Miller et al, 2005). RAND Corporation researchers proposed a detailed infrastructure for measuring structure, process, and outcomes of care in men with prostate cancer (Litwin et al, 2000; Spencer et al, 2003). This underpinned a national assessment of quality of care by applying specific quality indicators developed from the RAND work. Wide geographic variations exist in compliance with the RAND quality indicators; teaching/research hospitals and Comprehensive Cancer Centers have the highest compliance; racial differences are not observed for any indicator (Spencer et al, 2008). Early data suggest that radiation oncologists provide care that appears more compliant than that of urologists with the RAND quality indicators (Miller et al, 2007).
Structure of Care
Although certain structural characteristics may be necessary to provide good care, they are usually insufficient to ensure quality of care. Therefore the best structural measures are those that can be shown to have a positive influence on the process of care and on patient outcomes, although this relationship has not been confirmed (Brook et al, 1990). One structural measure that is positively associated with outcomes is the volume or number of cases treated by a particular physician or institution (Joshi and Miller, 2004; Joudi and Konety, 2004; Katz et al, 2004; Lee et al, 2004; Nuttall et al, 2004; Dibra et al, 2005; Killeen et al, 2005; Lyman et al, 2005; Vitale et al, 2005; Anger et al, 2007; Jeldres et al, 2008).
Evidence links surgical volume with outcomes across multiple disease conditions; however, the explanation and significance of this finding remains unclear. First noted in 1979 (Luft et al, 1979), the volume-outcomes relationship has been extensively explored for various surgical and medical conditions. An Institute of Medicine analysis of 27 diagnoses and procedures revealed that in general volume is associated with morbidity and mortality outcomes, but the magnitude of the relationship varies widely (Halm et al, 2002). Surgical volume has gained traction in health policy decisions as a marker of surgical quality (Cooperberg et al, 2009).
Patients who undergo urologic cancer surgery for prostate, bladder, kidney, or testis tumor are less likely to experience complications and mortality if their surgery is performed by a high-volume surgeon in a high-volume hospital (Joudi and Konety, 2004; Taub et al, 2004; Hollenbeck et al, 2007; Hollenbeck et al, 2007; Gilbert et al, 2008; Siu et al, 2008; Mayer et al, 2009). This observation also holds in men undergoing transurethral prostatectomy (Riley and Lubitz, 1986).
Patients treated at facilities or by surgeons who performed fewer radical prostatectomies experience more surgical complications than those treated by higher-volume providers (Lu-Yao et al, 1996; Ellison et al, 2000; Begg et al, 2002; Hu et al, 2003). Patients undergoing external beam radiation fare better when the radiation oncologist has a caseload of at least 10 per year or 200 cumulatively (Jeldres et al, 2008). It is not clear which characteristics of providers (surgeons or hospitals) performing many surgeries contribute to better outcomes; however, high volume appears to be an important predictor of good-quality care. As predicted with great prescience 3 decades ago by Harvard’s then-surgeon-in-chief, Dr. Francis D. Moore (Moore, 1980), regionalization of care for complex surgical procedures addresses disparities in quality. This has occurred for malignant and benign conditions in urology (Hollenbeck et al, 2005; Hollenbeck et al, 2006; Morris et al, 2006).
Process of Care
Process measures form the basis for most extant quality assurance efforts including the National Center for Quality Assurance’s Health Plan Employer Data and Information Set (HEDIS) and underlie most of the new “pay-for-performance (P4P)” incentives being pursued for reimbursement incentive systems (Epstein et al, 2004). However, quality assessment efforts should also incorporate structure and outcome measures. Birkmeyer and colleagues have suggested a framework in which quality metrics are driven by the risk a procedure poses to a patient and the average hospital caseload for the procedure (Birkmeyer et al, 2004). For high-risk, low-volume procedures such as nephrectomy with caval thrombectomy, structural measures are most appropriate because samples are too small to assess processes and outcomes. For high-risk, high-volume procedures such as radical prostatectomy, efforts may be more focused on processes and outcomes. The National Surgical Quality Improvement Program (NSQIP) developed by the U.S. Department of Veterans Affairs (VA) (Khuri et al, 1998) and under evaluation for non-VA hospitals (Fink et al, 2002) provides a framework for high-risk, high-volume procedures.
Outcomes of Care
Outcomes include changes in patients’ current and future health status including health-related quality of life and satisfaction. Cancer researchers generally use survival or progression-free survival as the main outcome measure in clinical studies. Sometimes proxy measures (also called surrogate end points or intermediate outcomes) that do not measure the outcome directly but are thought to be correlated with it are used. When a proxy measure is used as a quality indicator, there must be evidence that the proxy measure is truly a substitute for the outcome of interest. For example, rapid PSA velocity after treatment for localized prostate cancer appears to be associated with cause-specific mortality (Patel et al, 1997; D’Amico et al, 2005), so PSA doubling time may be a reasonable proxy outcome. Although the ultimate outcome may be mortality, many conditions in urology such as prostate cancer take an indolent course, making mortality impractical (and often irrelevant). In such conditions, more proximal outcomes such as length of stay, complications, and the need for salvage therapy provide a useful proxy (Hu et al, 2008). For proxy measures to be useful as quality measures, intervention should affect both the measure and the underlying disease (Schatzkin et al, 1996).
The most important patient-reported outcome is health-related quality of life, a multidimensional construct that includes somatic symptoms, functional ability, emotional well-being, social functioning, body image, as well as overall well-being (Guyatt et al, 1986; Cella and Bonomi, 1995; Guyatt et al, 1997). Quality of life assessment, typically by patient survey, provides a comprehensive evaluation of how the illness and its treatment affect patients.
Another patient-reported outcome commonly measured is patient satisfaction (Strasser et al, 1993), which refers to patients’ perceptions of the quality of care they received. Patient satisfaction is also usually assessed by patient survey. One limitation of satisfaction ratings is that patients are not generally able to evaluate the technical quality of their care. In fact, studies have found no consistent relationship between patient satisfaction and technical quality of care (Cleary and McNeil, 1988; Hayward et al, 1993). That is, a physician who interacts with patients in a warm and open way may provide care that is technically poor (Aharony and Strasser, 1993). In addition, patients’ satisfaction ratings may vary with their expectations. Nonetheless, when used in conjunction with other measures, data about patient satisfaction can provide useful information about overall quality of care.
Challenges to Using Outcomes to Evaluate Quality of Care
Outcomes can also be measured for more than one purpose. Although we are interested in developing outcome measures for evaluating the quality of care received by patients with urologic diseases, outcomes are also used clinically to track a patient’s progress and, in clinical trials, to measure the efficacy or effectiveness of a new drug or intervention (Williams et al, 2004). The same measures can sometimes be used for both purposes, but certain measures are better suited for one purpose or the other. For example, 5-year survival rates are a standard measure used in many studies of new cancer treatments. However, when measuring quality of care for purposes of accountability or quality improvement, we generally need a shorter time horizon than 5 years. If we compared the 5-year survival of patients with bladder cancer at two institutions, we might indeed find that one institution had higher survival rates, suggesting that it had better quality of care. However, during those 5 years, changes in staff, revamped procedures, or new technology may have improved or weakened the quality of care at the hospitals, thereby making the comparison of only historical relevance.
Health-Related Quality of Life
Health-related quality of life (HRQOL) is one of several variables commonly studied in the field of medical outcomes research. HRQOL encompasses a wide range of human experience including the daily necessities of life such as food and shelter, intrapersonal and interpersonal responses to illness, and activities associated with professional fulfillment and personal happiness (Patrick and Erickson, 1993). Contemporary interpretations of HRQOL are based on the World Health Organization’s longstanding definition of health as a “state of complete physical, mental, and social well-being and not merely the absence of disease” (WHO, 1948). Because illness may affect both quantity and quality of life, all constituents of well-being must be addressed when treating patients with urologic diseases. Perhaps most importantly, HRQOL involves patients’ own perceptions of their health and ability to function in life. Indeed, patient perceptions of physical function have prognostic value in predicting survival (Fossa, 1994). In light of evidence that survival and clinical outcomes may be similar across treatments for many conditions, quality of life considerations may be the critical factor in medical decision making for some instances.
Key Point
World Health Organization Definition of Health
Although quantity of life is relatively easy to assess in terms of survival, the measurement of quality of life presents more challenges, primarily because it is less familiar to most clinicians (Litwin, 1994; Meyer and Clayton, 2009). To quantify these qualitative phenomena, the principles of psychometric test theory are applied. This discipline provides the theoretic underpinnings for the science of survey research (Tulsky, 1990; Aaronson, 1991; Deyo et al, 1991; McSweeny and Creer, 1995; Testa and Simonson, 1996; Guyatt et al, 1997). Data are collected with HRQOL surveys, called instruments. Instruments typically contain questions, or items, that are organized into scales. Each scale measures a different aspect, or domain, of HRQOL. For example, items on a particular instrument may address a patient’s ability to have an erection and his satisfaction with ejaculation, both of which might be included in a sexual domain.
Some scales comprise dozens of items, whereas others may include only one or two items. Each item contains a stem (which may be a question or a statement) and a response set. Most response sets are one of the following types: (1) Likert scale, in which the respondent selects from a list of degrees of agreement or disagreement with the stem; (2) Likert-type scale, in which the respondent chooses from a list of text responses; (3) visual analog scale, in which the respondent marks a point on a line that is anchored on both ends by descriptors; and (4) numeric rating scale, in which the respondent chooses a number, usually between 0 and 10. Other response sets and approaches have been developed for children, people of low literacy, and various other populations (Nelson et al, 1990; Adler et al, 2000; Finlay and Lyons, 2001).
It is axiomatic that HRQOL assessments capture patients’ own perceptions of their health and ability to function in life. Instruments are best when they are self-administered by the patient, but if interviewer assistance is required, it must be from a neutral third party in a standardized fashion. Some studies have demonstrated that physicians typically underestimate the symptom burden experienced by prostate cancer patients, perhaps because their queries are not sensitive enough or because patients tend to understate their problems when speaking directly with the primary caregiver (Slevin et al, 1988; Fossa et al, 1990; Litwin et al, 1998b; Sonn et al, 2009). Other studies, however, suggest that physicians tend to overestimate the impact of the disease and its treatment on patients’ psychosocial functioning and sense of well-being (Fossa et al, 1996; Lampic et al, 1996; Sneeuw et al, 1997) Conversely, spouses may overstate some domains and understate others when compared with patient assessments (Sprangers and Aaronson, 1992). Kornblith et al (1994) presented results from a large sample of patients and spouses, both of whom were administered several validated HRQOL measures. Spouses reported greater psychologic distress but fewer sexual problems than did patients themselves. In a study of perspectives on HRQOL during antihypertensive therapy, Testa (1993) demonstrated that physicians were less sensitive to the impact of side effects, reporting less than 15% of the symptoms reported by patients. Spousal reports were more sensitive than patient self-assessments, particularly in the area of sexual functioning.
HRQOL Instruments
HRQOL instruments may be general or disease specific. General HRQOL domains address the components of overall well-being, whereas disease-specific domains focus on the impact of particular organic dysfunctions that may affect HRQOL. General HRQOL instruments typically address general health perceptions, sense of overall well-being, and function in the physical, emotional, and social domains. Disease-specific HRQOL instruments focus on special and/or more directly relevant domains such as anxiety about cancer recurrence, dizziness from antihypertensive medications, or suicidal thoughts during depression therapy (Patrick and Deyo, 1989). Disease-specific and general HRQOL domains often impact each other, leading to important interactions that must be considered in the interpretation of HRQOL data (Fossa et al, 1997). Further research is necessary in urology to explore how much of the variation in overall HRQOL is explained by variation in the disease-specific domains.
Numerous HRQOL instruments have been validated for use in urologic and other conditions. Many psychologists, sociologists, and statisticians devote their entire professional careers to the activity of developing and validating these instruments. Most medical research collaboratives devote substantial efforts to the development and standardization of HRQOL instruments (Moinpour et al, 1990; Sprangers et al, 1998). One medical journal, Quality of Life Research, is dedicated exclusively to presenting this research. Hence an abundance of literature exists on general HRQOL, and a significant body of work has been published on HRQOL in patients with various conditions (McDowell and Ewell, 1987; Patrick and Erickson, 1993). In urology, HRQOL research has been broad, but much has focused on individuals with prostate cancer, urinary incontinence, benign prostatic hyperplasia, end-stage renal disease, and bladder cancer (Edgell et al, 1996; Blaivas, 1998; Eton and Lepore, 2002; Botteman et al, 2003; Penson et al, 2003; Matza et al, 2004). A comprehensive resource for validated HRQOL instruments is available at www.proqolid.org. The National Cancer Institute has been particularly active in establishing interest in outcomes measurement for patients with malignant disease (www.outcomes.cancer.gov). Table 5–1 presents many of the validated health-related quality of life instruments available for the assessment of patients with urologic conditions. For each instrument, the table includes the number of items, recall time, and Flesh-Kinkaid reading grade level (Flesch, 1948; Kincaid et al, 1975).
Table 5–1 Characteristics of Health-Related Quality of Life (HRQOL) Instruments in Urologic Diseases
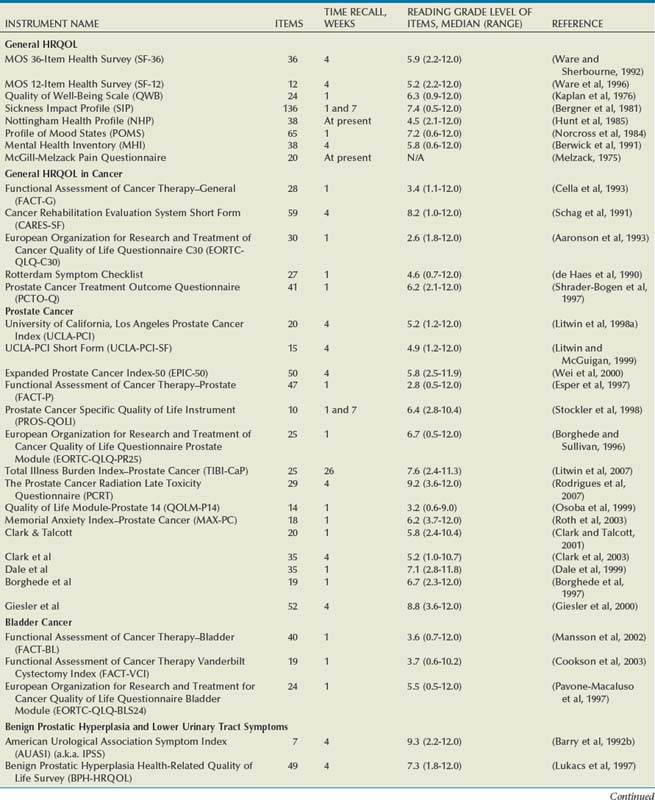
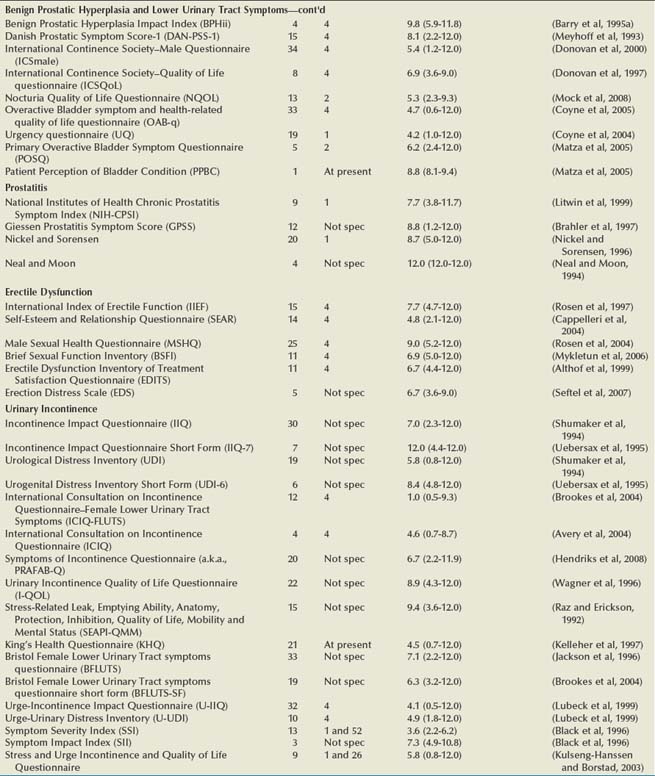
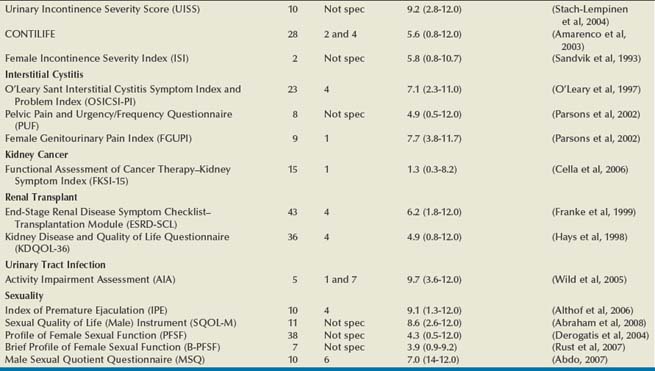
General HRQOL Instruments
General quality of life instruments have been extensively studied and validated in many types of patients, sick and well. Examples include the RAND Medical Outcomes Study 36-Item Health Survey, also known as the SF-36 (Ware and Sherbourne, 1992; Ware et al, 1994; Gandek et al, 1998b), the Quality of Well-Being scale (Kaplan et al, 1976; Kaplan and Bush, 1982; Kaplan and Anderson, 1988; Anderson et al, 1989; Kaplan et al, 1997; Kaplan et al, 1998), the Sickness Impact Profile (Bergner et al, 1976; Bergner et al, 1981), and the Nottingham Health Profile (Martini and McDowell, 1976; McDowell et al, 1978; Hunt et al, 1985). Each assesses various components of HRQOL including physical and emotional functioning, social functioning, and symptoms. Each has been thoroughly validated and tested for reliability, validity, and responsiveness.
The RAND Medical Outcomes Study 36-Item Health Survey (a.k.a., SF-36) is one of the most commonly used instruments and is regarded by some as a “gold standard” measure of general HRQOL. It is a 36-item, self-administered instrument that takes less than 10 minutes to complete and quantifies HRQOL in multi-item scales that address eight different health concepts—physical function, role limitation due to physical problems, bodily pain, general health perceptions, social function, emotional well-being, role limitation due to emotional problems, and energy/fatigue. The SF-36 may also be scored in two summary domains—physical and mental. Recently, a shorter 12-item version, the SF-12, has been developed for use in studies requiring greater efficiency. It provides a somewhat narrower view of overall health status and is usually scored only in the two summary domains (Ware et al, 1995; Ware et al, 1996; Gandek et al, 1998a). A useful resource for clinicians and researchers interested in the SF-36, SF-12, and related measures may be found at www.sf-36.org.
The Quality of Well-Being scale (QWB) summarizes three aspects of health status—mobility, physical activity, and social activity—in terms of QALY, quantifying HRQOL as a single number that may range from death to complete well-being. The original QWB contains only 18 items, but it requires a trained interviewer. A newer self-administered version of the QWB is now available and has been shown to produce scores that are equivalent to the interviewer-administered version and stable over time (Kaplan et al, 1997). The Sickness Impact Profile (SIP) measures health status by assessing the impact of sickness on changing daily activities and behavior. It is self-administered but contains 136 items and can take 30 minutes or longer to complete. The Nottingham Health Profile (NHP) covers six types of experience that may be affected by illness: pain, physical mobility, sleep, emotional reactions, energy, and social isolation by using a series of weighted yes or no items. It contains 38 self-administered items and can be completed fairly quickly.
Mental health is often measured with the Profile of Mood States (POMS) (Jacobson et al, 1978; Norcross et al, 1984; Cella et al, 1987; Albrecht and Ewing, 1989), a 65-item self-administered instrument that measures dimensions of affect or mood in six domains including anxiety, depression, anger, vigor, fatigue, and confusion. A validated short form is also available (Baker et al, 2002). Another commonly used measure of depression is the Center for Epidemiologic Studies Depression index (CES-D) (Weissman et al, 1977). Comprising items from previously developed scales, the CES-D includes 20 statements of depressive symptoms, which the respondent rates with a frequency ranging from rarely/none of the time to most/all of the time. A score of 16 or higher indicates a high likelihood of depression.
Another approach to quantifying general HRQOL is to blend a self-assessment of physical, emotional, and social functioning and well-being with a self-report of preferences, or utilities, for those health states. Developed by the Euroqol Group, a measurement collaborative, the EQ-5D is such an instrument (EuroqolGroup, 1990; Brooks, 1996; Johnson and Coons, 1998; Johnson et al, 1998; Sullivan et al, 2005). It is a short, generic measure of well-being in five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each of which is colored with a self-rating of no problems, mild/moderate problems, or severe problems. Responses are then weighted to yield a summary index, which ranges from worst to best possible health. Used extensively throughout the world, it was developed to incorporate patient preferences with the health states assessed by several existing generic instruments.
Benign Disease-Targeted HRQOL Instruments
The best-known outcomes instrument in urology is no doubt the American Urological Association Symptom Index (AUASI) (Barry et al, 1992a, 1992b; Barry et al, 1995a). Its simplicity belies its elegance and utility. Developed and published in the early 1990s, the AUASI revolutionized the longitudinal assessment of men with prostatic obstruction. For generations, urologists assessed men with prostatic enlargement by recording an inventory of obstructive and irritative voiding symptoms in qualitative categories such as 3+ hesitancy and nocturia ×2. Previous symptom indices (Barry et al, 1992a) never quite reached a tipping point, but with the imprimatur of the American Urological Association, the AUASI was rapidly and widely adopted as a quantitative measure of voiding symptoms (Gee et al, 1995
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






