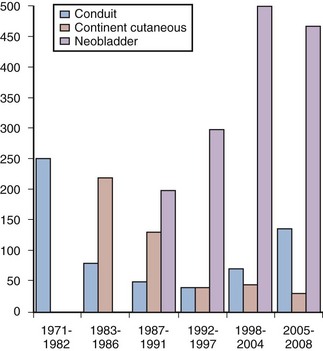
Eila C. Skinner, MD, Donald G. Skinner, MD, John P. Stein, MD Ureterosigmoidostomy is the oldest form of clinically applied urinary diversion. The first reported urinary diversion into a segment of bowel was by Simon in 1852. He attempted a ureterosigmoidostomy in an exstrophy patient by drawing the ureters into the rectum with the use of needles and suture to create a fistula. Although the patient died of sepsis 12 months later, this marked the first reported attempt at some form of urinary diversion (Simon, 1852). Over the following 100 years the evolution of urinary diversion was marked by a continued search for better methods and techniques to reconstruct the lower urinary tract. A number of technical modifications of the ureterosigmoidostomy ensued, particularly related to the ureteral implantation technique (Hinman, 1936). The rates of obstruction and ascending pyelonephritis in patients with ureterosigmoidostomy were significantly reduced after a direct anastomosis of the ureter into the sigmoid colon, incorporating an antireflux submucosal tunnel (Leadbetter, 1951; Goodwin et al, 1953). Ureterosigmoidostomy remained the diversion of choice until the late 1950s, but long-term electrolyte imbalance, upper tract obstruction and infection, and secondary malignant neoplasms arising at the ureteral implantation site were observed (Leadbetter and Clarke, 1954; Clarke and Leadbetter, 1955; Ridlon, 1963; Wear and Barquin, 1973). These significant complications inspired surgeons to develop better forms of urinary diversion. In 1950 Bricker refined and popularized the ileal conduit form of urinary diversion, building on an original description by Zaayer in 1911 (Zaayer, 1911; Bricker, 1950). The ileal conduit is a technically simple urinary diversion. This form of reconstruction was a reliable technique for urinary diversion and became widely accepted. It has remained the “gold standard” to which other types of urinary diversion are compared. It continues to be the most common form of urinary diversion performed throughout the world today. Long-term complications with the Bricker ileal conduit started to come to light in the 1970s. Although problems with hyperchloremic metabolic acidosis and pyelonephritis were substantially less common than in patients with ureterosigmoidostomy, late complications with the ileal conduit such as stomal stenosis, pyelonephritis, calculus formation, ureteral obstruction, and renal deterioration became more apparent with longer follow-up (Butcher et al, 1962; Shapiro et al, 1975; Middleton, 1976; Johnson and Lamy, 1977; Pitts and Muecke, 1979; Sullivan et al, 1980). These clinical sequelae were thought to be related to the reflux of infected urinary or obstruction of the upper urinary tract. It was postulated that the addition of an antireflux technique to a conduit form of diversion could help diminish the problems of reflux and renal deterioration in these patients. In fact, well-performed laboratory experiments provided evidence to support the advantage of nonrefluxing colonic conduits over ileal conduits (Richie et al, 1974; Claesson et al, 1985). Unfortunately, with longer follow-up, similar complications with colon conduits including upper urinary tract damage developed, again dampening enthusiasm for this form of urinary diversion (Morales and Golimbu, 1975; Althausen et al, 1978; Elder et al, 1979). The first continent diversion was described by Gilchrist and colleagues. This form of urinary reconstruction incorporated a cecal reservoir with the ileocecal valve as the continence mechanism and the distal ileum as a catheterizable stomam (Gilchrist et al, 1950). However, this innovation attracted little attention at the time. The concept of a continent cutaneous diversion was subsequently reintroduced by Kock and colleagues (1982). This diversion was originally designed for a continent terminal ileostomy and used an intussuscepted nipple valve to maintain continence and avoid reflux. In animal experiments and then in humans, Kock demonstrated the importance of complete detubularization of the bowel segment and the double-folding technique that creates the most spherical shape possible (Eckman et al, 1964; Kock et al, 1982). These concepts are the cornerstone of current cutaneous and orthotopic reservoirs. After Kock described his results in his initial 12 patients, Donald Skinner began performing this diversion in adults undergoing cystectomy for bladder cancer. Although this form of urinary diversion required catheterization of an abdominal stoma, it eliminated the need for and associated problems with an external urostomy appliance. It was a popular concept for patients and referring physicians alike, and Skinner quickly amassed a large clinical experience with this type of diversion (Skinner et al, 1984, 1987). The “Achilles’ heel” of continent cutaneous reservoirs is the design of a reliable, durable, efferent continence mechanism that is easily catheterizable. A number of techniques have been described using large and small bowel and even stomach, with many ingenious continence mechanisms. However, stones, difficulty catheterizing, peristomal hernias, and the development of leakage are potential problems with all of them, often requiring open surgical revision to resolve (Lieskovsky et al, 1987; Rowland, 1995). The concept of orthotopic diversion began even before Gilchrist’s continent cutaneous diversion. Tizzoni and Poggi (1988) were the first to experiment in a dog transplanting the ureters into an isolated loop of ileum interposed between the ureters and the urethra. The dog was reportedly continent and subsequently underwent three successful pregnancies before expiring 30 months postoperatively (Tizzoni and Poggi, 1988). Lemoine is credited with performing the first orthotopic reconstruction in a human subject. This patient initially underwent a cystectomy with ureterosigmoidostomy. Complications related to recurrent pyelonephritis led to undiversion in this patient; the rectal segment was isolated, transected, and anastomosed to the urethra, and the sigmoid colon was brought down and anastomosed to the anus (Lemoine, 1913). In 1979 Camey and Le Duc reported their pioneering and extensive clinical experience with orthotopic substitution to the native urethra in male bladder cancer patients (Camey and Le Duc, 1979). This was a substantial accomplishment that demonstrated the feasibility of lower urinary tract reconstruction to the native urethra. The initial Camey diversion used an intact segment of ileum, resulting in a high-pressure reservoir. Subsequently the Camey II detubularized reservoir (Camey, 1990); the Hautmann W-neobladder (Hautmann et al, 1988); the Studer pouch (Studer et al, 1989); the T pouch (Stein et al, 1998b); stomach neobladder (Hauri, 1998); cecal and ileocecal neobladders (Light and Englemann, 1986; Mansson and Colleen, 1990); and sigmoid reservoir (Reddy and Lange, 1987) have been described. Many of these now have a large clinical experience with long-term follow-up demonstrating good renal preservation and continence results (discussed further later). Initially these techniques were only applied to male patients because continence in the female was believed to be dependent on an intact bladder neck. In the mid-1990s it was discovered through anatomic dissections and initial clinical experience that women could remain continent with a low-pressure reservoir and preservation of only the urethra itself (Borirakchanyavat et al, 1997; Colleselli, 1998). In addition there were several careful pathologic studies of female cystectomy specimens showing that preservation of the urethra was safe in the majority of women with bladder cancer without compromising the oncologic efficacy of the operation (Stein et al, 1995, 1998a; Stenzl, 1995b; Maralani, 1997). Although the ideal bladder substitute remains to be developed, the orthotopic neobladder most closely resembles the original bladder in both location and function. This form of lower urinary tract reconstruction relies on the intact external rhabdosphincter continence mechanism, usually does not require intermittent catheterization, and avoids the difficulties associated with the efferent continence mechanism of continent cutaneous reservoirs. Voiding is accomplished by concomitantly increasing intra-abdominal pressure (Valsalva maneuver) with relaxation of the pelvic floor musculature. The majority of patients undergoing orthotopic reconstruction are continent and void to completion without the need for intermittent catheterization. The pioneering work of Camey and Le Duc with orthotopic reconstruction in carefully selected male patients has subsequently evolved into a common form of lower urinary tract reconstruction potentially applicable to most patients requiring urinary diversion (Camey and Le Duc, 1979). In many centers worldwide orthotopic reconstruction has replaced the ileal conduit as the standard form of reconstruction. The experience of urinary diversion at the Keck University of Southern California School of Medicine demonstrates this evolution (Fig. 87–1). Beginning in 1986, the number of orthotopic bladder substitutes performed dramatically increased while the number of conduits dramatically declined. Currently the authors perform continent orthotopic diversion in approximately 90% of male and 75% of female patients undergoing radical cystectomy. It is, however, incumbent on the surgeon who is actively involved in lower urinary tract reconstruction to understand the indications for and contraindications to orthotopic diversion, as well as to be familiar with the various reconstructive options. This will help ensure optimal clinical outcomes and improved satisfaction of patients. Key Points: Historic Evolution of Orthotopic Urinary Diversion Secondly, the reservoir must be sufficiently compliant to maintain a low pressure throughout the filling phase. This is best achieved by splitting the bowel segment open longitudinally to completely detubularize it and folding it to create a spherical shape. This concept was described by Goodwin and colleagues (1959) and further developed by Kock in elegant animal experiments (Eckman et al, 1964). The sphere has the greatest internal volume for a given surface area and thus the greatest capacity. By Laplace’s law (T = PR) for a given wall tension (T), the internal pressure (P) decreases as the radius (R) of the sphere increases. The compliance of the wall relates to the physical characteristics of the bowel wall itself and is greater in small bowel compared with large bowel. The double-folded technique of Kock, or S– or W-shaped reservoirs all effectively ablate the coordinated high-pressure contractions of the bowel wall, allowing the reservoir to maintain low internal pressure throughout the filling phase (Kock et al, 1982; Hinman, 1988). Early techniques for bladder augmentation using an intact cecal segment and the early Camey orthotopic ileal neobladder failed to incorporate the important step of detubularization and thus resulted in unacceptable incontinence and upper tract deteriorization (Camey and Le Duc, 1979; Lilien and Camey, 1984). All current techniques use detubularized bowel to construct the reservoir portion of the orthotopic diversion. Thirdly, the reservoir must have adequate volume to allow for reasonable voiding intervals. This generally should be at least 300 to 500 mL. All bowel segments effectively stretch over time if there is adequate outflow resistance. The standard 44-cm length of ileum formed into a double-folding reservoir by the Kock technique (also used for both the Studer and T pouch neobladders) holds less than 200 mL initially. When used for a cutaneous pouch with high outflow resistance, this segment routinely stretches up over time to hold more than 1000 mL at low pressure. Such high volumes are not ideal for orthotopic diversion because of the difficulty emptying such large reservoirs, so patients are encouraged to empty more frequently. Nevertheless, this emphasizes that larger initial volumes are not necessary to ultimately achieve an adequate voiding volume. However, colonic segments do not stretch up as easily and a larger initial volume may be necessary for pouches constructed out of colon. In general small bowel, when available, has advantages over colon in terms of wall compliance and ability to stretch, as well as reduced mucous formation (Khafagy, 2006). Key Points: Three Basic Principles of Orthotopic Neobladder Construction The primary cancer-related contraindication for orthotopic diversion is presence of urothelial carcinoma in the urethral stump to which the neobladder is to be connected. In the male patient involvement of the prostatic urethra is associated with a higher risk of subsequent urethral recurrence. Ashworth first reported that patients with prostatic urethral involvement are at increased risk for urethral tumor involvement after cystectomy and ileal conduit. Prostatic urethral tumor involvement was found in five of seven patients (71%) who later developed anterior urethral tumors (Ashworth, 1956). Other series have subsequently confirmed this finding (Raz et al, 1978; Faysal, 1980; Hardeman, 1990; Levinson et al, 1990; Tobisu et al, 1991; Nieder et al, 2004). Freeman and colleagues (1994) reviewed six studies, in which 31 of 122 patients (25%) with some form of prostatic urethral involvement developed an anterior urethral tumor after radical cystectomy for bladder cancer. These findings have subsequently been confirmed in our cystectomy series in which the overall probability of urethral recurrence was estimated to be approximately 7% at 5 years and 9% at 10 years. Recurrences were observed at a median of 2 years after cystectomy (range 0.2 to 13 years). The risk was only 5% at 5 years in the 639 patients without any prostate tumor involvement compared with 11% for the 129 men with any prostate involvement (Stein et al, 2005). In a large, comprehensive, pooled analysis of 25 series, Stenzl and colleagues (1995b) reported a total of 256 anterior urethral tumor recurrences in 3165 patients (8.1%) undergoing cystectomy for bladder cancer. Specific characteristics of the primary bladder cancer have been analyzed to determine if any particular histopathologic parameters can identify patients at increased risk for urethral recurrence after cystectomy. Various pathologic risk factors have been implicated including the presence of papillary tumors, tumor multifocality, trigone or bladder neck tumor involvement, associated carcinoma in situ in the bladder or upper tracts, and various degrees of involvement of the prostate (Freeman et al, 1996). Several investigators have evaluated the presence of carcinoma in situ in the bladder as a risk factor for urethral recurrence with variable results (Hardeman and Soloway, 1990; Levinson et al, 1990; Tobisu et al, 1991; Stein et al, 2005). Tobisu and colleagues (1991) combined these pathologic risk factors and showed in a multivariate analysis that the risk of urethral recurrence more than doubled each time the number of risk factors was increased by one. Of all the risk factors, the degree of prostatic tumor involvement is most useful in predicting the risk of subsequent urethral recurrence. In general, prostatic involvement can be divided into urethral mucosa only (including carcinoma in situ), ductal involvement with carcinoma in situ, and prostatic stromal invasion. Hardeman and Soloway (1990) evaluated 30 patients with some involvement of the prostate and found anterior urethral recurrence in 11 (37%). No patient with only mucosal involvement developed a recurrence, whereas 25% of men with prostatic ductal involvement and 64% of men with prostatic stromal involvement developed urethral recurrence. In the same series, only 2 of 56 patients (4%) without prostatic urethral tumor involvement in the primary tumor suffered subsequent urethral recurrence (Hardeman and Soloway, 1990). In another study, Levinson and colleagues (1990) found similar results, with urethral recurrences rates of 0% for mucosa only, 10% for ductal involvement, and 30% with stromal invasion. Overall, 67% of their patients with urethral recurrences had a history of prostatic urethral involvement in their primary tumor (Levinson et al, 1990). Hassan and colleagues (2004) reported an even lower risk of urethral recurrence, with only one recurrence observed in more than 400 patients, though with relatively short follow-up. These observations were confirmed in our series of 768 men undergoing cystectomy without urethrectomy with long-term follow-up (median 13 years) (Stein et al, 2005). The extent of prostatic tumor involvement was the most significant predictor for urethral tumor recurrence. Of the 129 patients with pathologic transitional cell carcinoma involving the prostate at cystectomy, 14 (11%) developed urethral recurrence. The 5-year estimated probability of urethral recurrence for superficial (mucosa and ductal, without stroma) involvement was 12%, compared with 18% with prostatic stroma invasion. In a multivariate analysis, any prostate tumor involvement (superficial or invasive) remained an independent and significant predictor of a urethral tumor recurrence. Carcinoma in situ and tumor multifocality were not individually associated with a significant risk for anterior urethral recurrence (Stein et al, 2005). Collectively, these studies confirm that prostatic stromal invasion is the single strongest pathologic predictor of subsequent recurrence in the anterior urethra after cystectomy for bladder cancer. It does not appear that the presence of carcinoma in situ in the bladder alone is a clear risk factor for subsequent urethral recurrence. Some evidence indicates that orthotopic diversion itself may provide some protection against urethral recurrence. In the University of Southern California experience, the type of diversion was an independent variable predicting urethral recurrence. In patients without prostatic involvement, the risk of urethral recurrence at 5 years was 3% versus 8% in patients with orthotopic versus cutaneous diversion, respectively. Interestingly, there was no benefit seen if patients with any prostatic involvement were taken as a whole. However, in patients with the highest-risk disease (prostatic stromal invasion) there did seem to be some protection for those undergoing orthotopic diversion, with a 5-year risk of recurrence of 11% versus 24% for those with cutaneous diversion. The reason for this observation is unclear, though there has been speculation that continued flow of urine, perhaps with changes in urinary characteristics due to the interposed bowel, might be responsible (Stein et al, 2005). There is some controversy about the importance of attempting to identify prostatic urethral involvement preoperatively, as well as what to recommend for those patients in whom prostatic involvement is identified. At the time of transurethral resection of the primary bladder tumor, the surgeon may take deep transurethral resection biopsies of the prostate, preferably at the 5- and 7-o’clock positions lateral to the verumontanum. This should certainly be done if the mucosa of the prostate looks suspicious or in cases with obvious tumor at the bladder neck. Some authors have recommended that this should be done routinely and have advocated repeat transurethral resection (TUR) before cystectomy in cases where it was omitted (Wood et al, 1989; Sakamoto et al, 1993). However, the reliability of preoperative transurethral prostatic biopsies has been challenged by others (Lebret et al, 1998; Donat et al, 2001). In a prospective series of 118 patients, Lebret and colleagues examined the utility of preoperative prostatic biopsies compared with intraoperative frozen-section analysis of the prostatic urethral margin at the time of cystectomy in predicting urethral recurrence. They found that intraoperative frozen-section analysis was more accurate than any preoperative parameter including preoperative prostate biopsies in predicting urethral recurrence (Lebret et al, 1998). In another series of 246 men who underwent preoperative transurethral loop biopsy of the prostate, Donat and colleagues (2001) reported that this preoperative pathologic evaluation did not accurately determine prostatic tumor involvement—both false-negative and false-positive results were observed. Forty-three percent of patients with prostatic involvement on final pathology were missed on the TUR biopsy, and 12 of 36 patients with prostatic stromal invasion identified on TUR specimen had no residual prostatic involvement on the final cystectomy specimen (Donat et al, 2001). In light of this it is questionable whether the risk of the additional anesthetic and the potential delay to definitive surgery warrants the additional information garnered by a repeat TUR with prostatic urethral biopsy. The authors have not routinely recommended repeat TUR before cystectomy in the majority of patients. Other centers have adopted a similar philosophy with comparable clinical outcomes (Iselin et al, 1997; Hautmann, 2003). It has been the authors’ practice to counsel patients with documented prostatic mucosal, ductal, or stromal invasion about the increased risk of urethral recurrence if the urethra is left in situ and to help them weigh that risk against any perceived advantage of an orthotopic diversion. If an orthotopic diversion is still preferred, the authors will depend on the intraoperative frozen section of the urethral margin to make the final decision. Patients with extensive prostatic stroma invasion, similar to those with clinical extravesical disease, should also be strongly considered for neoadjuvant chemotherapy before cystectomy (Grossman et al, 2003). If the patient opts for a cutaneous diversion, the authors will perform an en bloc urethrectomy at the time of cystectomy. In the past, urethrectomy was routinely performed at the time of radical cystectomy in women. With the acceptance of continent neobladder in men a number of investigators began evaluating the feasibility of preservation of the urethra in women. This was initially attempted in women with nontransitional cell carcinoma in whom there was little concern about possible urethral recurrence with excellent functional results (Stein et al, 1996). At the time it was generally believed that the bladder neck was the primary continence mechanism in women, so it was somewhat surprising that one could achieve excellent continence dividing distal to the bladder neck (Tanagho et al, 1966). Two important pathologic studies were critical to the ultimate decision to expand urethral-preserving cystectomy to women with urothelial carcinoma. Stein and colleagues (1995) retrospectively evaluated a series of archival cystectomy specimens from female patients undergoing cystectomy for bladder cancer. Sixty-seven consecutive female cystectomy specimens removed for transitional cell carcinoma of the bladder were pathologically re-evaluated. Histologic evidence of tumor involving the urethra was found in 9 women (13%), whereas 17 patients (25%) demonstrated bladder neck tumors. All female patients with an uninvolved bladder neck also had an uninvolved urethra (no skip lesions), whereas approximately 50% of patients with a bladder neck tumor had concomitant urethral tumor involvement. Risk factors for urethral involvement in this study included increased grade, stage, and lymph node involvement, but the presence of carcinoma in situ did not predict urethral involvement. Vaginal wall involvement was also a major risk factor for urethral involvement. Although vaginal wall invasion was a relatively rare event (1%), all of these patients also had bladder neck involvement and 50% had urethral extention (Stein et al, 1995). Stenzl and colleagues (1995b) also studied the risk of synchronous or secondary urethral tumors with long-term follow-up in women with bladder cancer. The charts of women treated for various stages of bladder cancer during a 19-year period were reviewed. They evaluated 356 women with a mean follow-up of 5.5 years. Overall, 7 of 356 patients (2%) had a urethral tumor at presentation (Stenzl et al, 1995b). This is a similar incidence as had been observed by Ashworth in his earlier endoscopic analysis. Of the women with clinical stages T2 to T3b, N0, M0 (potential candidates for radical cystectomy with preservation of the urethra), only 1% had urethral tumor (Ashworth, 1956). The authors’ evaluation of the impact of tumor localization revealed that bladder neck involvement was most significantly associated with secondary urethral tumor in these women (Stenzl et al, 1995b). Furthermore, no patient in this analysis had an isolated urethral tumor without concomitant bladder neck tumor involvement. The authors emphasized that the only consistent risk factor for urethral tumor involvement was concurrent tumor at the bladder neck. No correlation between urethral tumors and other pathologic factors such as carcinoma in situ or tumor multifocality was found. They concluded that the urethra can be safely preserved in selected female cystectomy patients provided that neither preoperative biopsy specimens of the bladder neck nor intraoperative frozen-section specimens of the proximal urethra demonstrate any tumor or atypia (Stenzl, 1995b). It has been suggested that the apparent lower incidence of urethral tumors in women (compared with men) may be related to the fact that transitional cell mucosa in women covers a smaller urethral segment, with the remainder being normal or metaplastic squamous cell mucosa. The area at risk in the female urethra is therefore smaller and probably diminishes with increasing age as the demarcation line between squamous and transitional cell mucosa migrates cranially during menopause. In the sixth and seventh decades of life, when most bladder tumors occur, metaplastic squamous cell mucosa may cover the entire urethra, the bladder neck, and even a portion of the trigone (Peckhman, 1971). This pathologic finding was confirmed by Maralani and colleagues (1997), who retrospectively evaluated 43 female cystectomy specimens removed for bladder cancer. They reported a 16% incidence of urethral tumor involvement, and vaginal involvement in this study was the most significant risk factor for urethral tumor involvement (Maralani et al, 1997). Similarly, Chen and colleagues also retrospectively reviewed the risk of secondary urethral, vaginal, and cervical involvement by transitional cell carcinoma in women undergoing radical cystectomy. They found an overall 8% incidence of tumor of the urethra, and approximately 50% of patients with vaginal or cervical invasion also demonstrated urethral tumor involvement. They similarly confirmed previous reports that the most significant risk factor for urethral tumor involvement is tumor at the bladder neck (Chen et al, 1997). Similar conclusions had been previously reached by Coloby and da Paepe (De Paepe et al, 1990; Coloby et al, 1994). Stein and colleagues (1998a) embarked on a prospective study to evaluate and confirm the previously established pathologic risk factors in women undergoing cystectomy for bladder cancer to determine if these criteria safely identify appropriate female candidates for orthotopic diversion. Final pathologic analysis of the bladder neck and proximal urethra was performed and compared with the intraoperative frozen-section analysis of the distal surgical margin (proximal urethra). Tumor involvement at the bladder neck and proximal urethra was found in 14 (19%) and 5 (7%) cystectomy specimens, respectively. All patients with urethral tumors also demonstrated concomitant bladder neck tumors. Bladder neck tumor involvement was again found to be the most significant risk factor for tumor involving the urethra, confirming the findings from retrospective series (Stein et al, 1995). However, approximately half of patients with bladder neck tumors had a normal (tumor-free) proximal urethra. Furthermore, no patient with a normal bladder neck demonstrated tumor involvement of the urethra. In all cases, intraoperative frozen-section analysis of the proximal urethra correlated with and was correctly confirmed by final permanent section (Stein et al, 1998a). These results were virtually identical to the results from previous retrospective study (Stein et al, 1995) and suggest that preoperative biopsy of the bladder neck or urethra is not necessary, but rather one may depend on the intraoperative frozen section to determine the feasibility of orthotopic diversion. Clinical follow-up data of the first 88 women undergoing orthotopic diversion at that institution showed that no urethral recurrences were observed with a median follow-up of 30 months in this group (Stein et al, 2002). More recently Al el Dein and colleagues (2004) prospectively evaluated 145 women undergoing cystectomy and orthotopic diversion. Two patients developed urethral recurrence. One had a primary squamous cell cancer of the bladder, and the other had urothelial carcinoma with carcinoma in situ of the trigone (Al el Dein et al, 2004). Radical cystectomy with bilateral pelvic iliac lymphadenectomy provides excellent local (pelvic) control for the treatment of invasive bladder cancer. Stein and colleagues (2001a) reported the clinical outcomes for 1054 patients who underwent radical cystectomy for bladder cancer with a median follow-up of more than 10 years. In this series, an overall local pelvic recurrence rate of 7% was observed for the entire group of patients. The risk of recurrence ranged from 6% with organ-confined, node-negative disease to 13% for patients with extravesical or node-positive disease (Stein et al, 2001a). These results suggest that local recurrence even for patients demonstrating locally advanced or lymph node–positive disease is relatively infrequent and should not necessarily preclude an orthotopic form of urinary diversion. In addition, the results of this study show that a significant percentage of patients with locally advanced disease will be long-term survivors and may benefit from a continent form of urinary diversion. Nearly 50% of patients with extravesical tumor extension and 30% of patients with lymph node–positive disease were still alive without evidence of disease 5 years following cystectomy (Stein et al, 2001a). If local tumor recurrence does develop in patients with an orthotopic diversion, only a minority will develop problems related to the urinary diversion itself. Hautmann and colleagues evaluated this question in 43 of 357 men who underwent radical cystectomy and ileal neobladder and developed local recurrence. Most of them (84 %) had advanced disease (≥pT3a) on final pathology at the time of cystectomy. A total of 17 patients (43%) had concomitant distant metastasis at the time of diagnosis of the local recurrence. Local recurrence interfered with the upper urinary tract in 24 cases, the neobladder in 10 (23%), and the intestinal tract in 7; only 1 patient required removal of the neobladder because of an intestinal fistula. The authors concluded that most patients can anticipate normal neobladder function even in the presence of locally recurrent disease (Hautmann and Simon, 1999). Similar results have been reported by others. Tefilli and colleagues (1999) found that 1 of 11 patients with orthotopic diversion required conversion to an ileal conduit after local recurrence. To evaluate the impact of orthotopic diversion on the quality of the cystectomy and ensuring appropriate cancer control, Yossepowitch and colleagues retrospectively evaluated 214 patients who underwent radical cystectomy and orthotopic reconstruction and compared them with 269 patients similarly treated with an ileal conduit diversion. Adjusting for pathologic stage, there was no cancer-specific survival difference between the two diversion groups. Patterns of relapse in 62 of the 214 patients (29%) with an orthotopic neobladder included local recurrence in 11%, distant recurrence in 9%, and combined local and distant recurrence in 18%. Only one patient in this series required the neobladder to be converted to an ileal conduit secondary to a relapse at the ureteroenteric anastomosis and expanding into the pouch. The authors concluded that the low risk of local recurrence showed that in this cohort of patients the oncologic efficacy of the operation was not compromised (Yossepowitch et al, 2003). Key Points: Cancer-Related Factors in Patient Selection Many authors have evaluated the success of continent diversion in elderly patients (Lance et al, 2001; Clark et al, 2005; Sogni et al, 2008). Although elderly patients undergoing orthotopic diversion may regain continence more slowly and have a higher rate of mild stress incontinence, ultimately older patients achieve daytime and nighttime continence rates similar to those for younger patients (Elmajian et al, 1996). The clear consensus is that age alone is not a contraindication for continent diversion and options should be considered for each patient on the basis of other factors. Medical comorbidities; renal, cardiac, pulmonary, and cognitive function; and manual dexterity are all important factors that should be considered, along with the patient’s social support situation. A frail, sedentary, elderly person is probably best served with an expeditious conduit. Similarly, a conduit may be easier for a caregiver to manage than an orthotopic diversion with the risk of incontinence. However, an active, generally healthy, elderly patient may certainly be considered a reasonable candidate for orthotopic diversion depending on his or her wishes. One of the most important contraindications for continent neobladder reconstruction is compromised renal function. Urinary electrolytes including urea, potassium, and bicarbonate are reabsorbed from the small bowel mucosa, resulting in an increased acid load that must be handled by the kidneys. In patients with compromised renal function, hyperchloremic metabolic acidosis can develop along with worsening dehydration, uremia, nausea, and bone loss. The exact level of acceptable renal function for consideration for continent diversion is somewhat controversial. As a general rule, a serum creatinine level of less than 1.7 to 2.2 mg/dL (150 to 200 µmol/L) or an estimated creatinine clearance of greater than 35 to 40 mL/min is recommended (Hautmann, 2003; Hautmann et al, 2007). However, decision making in individual patients can at times be less clear-cut. Acute upper tract obstruction caused by the tumor often results in a transient rise in creatinine, which would be expected to improve after cystectomy. The need to do occasional or routine self-catheterization is reported in 10% to 50% of men and in up to 30% to 50% of women (Hautmann et al, 2007). It is generally impossible to predict which patients will require catheterization to empty, and retention can occur many years after the initial surgery. Thus all patients considered for continent diversion should be willing and able to do self-catheterization. The authors have each patient meet with a specially trained enterostomal therapist before surgery to go over this technique in addition to other perioperative issues. It has been rare for a patient to decide against a continent orthotopic diversion because of this requirement. It is not uncommon for patients to present for surgical management of invasive bladder cancer after previous failed radiation therapy or after radiation treatment of a previous pelvic malignancy. Stein and colleagues (2001a) found that 8.5% of 1471 patients had some prior pelvic radiation. This exposure can significantly increase the difficulty of the surgery and can affect wound healing and postoperative complications. Many surgeons are hesitant to offer orthotopic diversion in this setting. Ahlering and colleagues (1988) retrospectively evaluated patients undergoing cystectomy and cutaneous continent diversion comparing 44 patients who had a history of prior radiation with 42 matched patients who did not. They found no significant difference between irradiated and nonirradiated patients in operative time, blood loss, transfusion requirements, and wound or ureteral complications (Ahlering et al, 1988). Kim and Steinberg found an increased risk of surgical complications, especially those that required percutaneous or surgical intervention, in 23 patients undergoing cystectomy and conduit following radiation compared with 23 matched controls (Kim and Steinberg, 2001). Chang and colleagues (2004) evaluated outcomes of ileal conduits in 36 patients with prior radiation. They found ureteroileal complications occurred in 9% of patients by 5 years and concluded that it was appropriate to use ileum for diversion rather than a colon conduit. The complications and ultimate functional outcome of orthotopic neobladders in patients with prior pelvic radiation have been evaluated by a number of authors. Gschwend and colleagues reported on 11 such patients. The postoperative course in this group including duration of hospital stay, perioperative complications, and early functional results did not differ from that of a control group of nonirradiated patients. They concluded that high-dose pelvic irradiation should not be a primary contraindication for orthotopic urinary diversion with segments of small intestine (Gschwend et al, 1996). Gheiler and colleagues (1997) evaluated their clinical outcomes in three patients undergoing orthotopic substitution after cystoprostatectomy for radiorecurrent prostate cancer. Postoperative complications included pyelonephritis in one patient and prolonged ileus in another. All patients with an orthotopic neobladder were continent during the day; one patient required a single pad at night. This group concluded from their small series that orthotopic urinary diversion is a valid option for well-selected patients with radiorecurrent prostate cancer who require salvage cystoprostatectomy and that it can be performed with minimal complications, resulting in good continence and quality of life (Gheiler et al, 1997). Bochner and colleagues (1998) described their experience with salvage surgery and orthotopic bladder substitution after failed radical radiation therapy. A total of 18 patients who had failed definitive radiation therapy (total minimum dose, ≥60 Gy) for bladder or prostate cancer and had undergone a salvage procedure with construction of an orthotopic neobladder were evaluated. Operative characteristics, postoperative outcomes, and complications (related or unrelated to the urinary diversion) were found to be similar in irradiated and nonirradiated patients. Good daytime and nighttime continence after surgery was reported by 67% and 56% of irradiated patients, respectively. Patients with poor postoperative continence (22%) were successfully treated with the placement of an artificial urinary sphincter. These authors concluded that salvage surgery and orthotopic urinary reconstruction appear to be safe, effective procedures that provide a functional lower urinary tract in patients in whom definitive pelvic radiation therapy has failed (Bochner et al, 1998). Nieuwenhuijzen and colleagues (2004) reported on 27 patients who underwent salvage cystectomy, 9 of whom had an orthotopic diversion. Eight of them achieved complete daytime continence, similar to results expected in nonirradiated patients (Nieuwenhuijzen et al, 2004). It is clear that in carefully selected patients, orthotopic lower urinary tract reconstruction can be performed after definitive, full-dose pelvic irradiation. Even selected women with a history of pelvic irradiation may be appropriate candidates for orthotopic reconstruction with good clinical outcomes (Stein et al, 2002; Lee et al, 2004). However, these are challenging procedures that clearly require technical expertise and keen intraoperative judgment. Previous high-dose prostate radiation (external beam or brachytherapy) or a vaginal implant for cervical cancer cause more scarring around the rhabdosphincter area than does radiation for either bladder cancer or previous colon cancer. Similarly, interstitial seed implants for prostate cancer may result in severe scarring around the area of the external sphincter, depending on the placement of the seeds. Preoperative evaluation including cystoscopy is mandatory to evaluate the integrity of the mucosa around the area of the sphincter. However, it may not be possible to accurately predict the degree of radiation damage found at surgery, so careful intraoperative tissue assessment and determination of the condition of the urethra, ureters, and bowel must be performed to make a final decision about the feasibility of orthotopic diversion (Abbas et al, 2001). These patients should always be counseled preoperatively that the orthotopic diversion may not be possible. Prior abdominal or pelvic surgery may also present challenges for the surgeon performing orthotopic diversion. Patients who have had a prior radical prostatectomy may have a particularly difficult dissection around the proximal urethra at the prior vesicourethral anastomosis. Nevertheless, this is often feasible with careful dissection. The health of the proximal urethra can be assessed at surgery and acceptable continence can be obtained in selected patients (Schuster et al, 2003). In general, with careful dissection a patient who was continent following the first surgery can be expected to have an acceptable result with a neobladder. Key Points: Previous Pelvic Radiation or Pelvic Surgery Much of what has been learned of the rhabdosphincter complex comes from elegant neuroanatomic studies of the female urethra. Colleselli and colleagues (1998) performed extensive microneuroanatomic dissections, histologic examination, and three-dimensional reconstructive imaging to better define the urethral sphincteric and rhabdosphincteric anatomy in women. The female urethral sphincter system consists of smooth muscle innervated by the autonomic nervous system and striated muscle supplied by somatic nerves. There is general agreement that the autonomic nerves that serve the smooth muscle sphincter originate in the pelvic plexus. These autonomic fibers emerge from the the pelvic plexus and course along the lateral aspect of the rectum and vagina toward the bladder neck and very proximal urethra. Some of these fibers branch off from a thick fiber at the lower margin of the lateral vaginal wall and enter the bladder neck and cranial portion of the urethra from the dorsolateral aspect. These autonomic nerves do not appear to play a significant role in the innervation of the rhabdosphincter or the continence mechanism and are essentially sacrificed during the exenterative portion of the operation without compromising continence (Colleselli et al, 1998). Innervation of the voluntary urinary sphincter system, however, is a matter of some controversy. Most interested investigators agree that the rhabdosphincter is probably supplied primarily by the branches of the pudendal nerve (Borirakchanyavat et al, 1997; Stenzl et al, 1997; Colleselli et al, 1998). Although these dissections were performed on female cadavers, the observations and findings have been similarly described in men (Strasser, 2000). Collectively, these findings have allowed a more precise and anatomic approach to maintain the continence mechanism in all patients undergoing cystectomy and orthotopic substitution. In the same study, Colleselli and colleagues (1998) found that the major portion of the striated muscle that corresponds to the striated rhabdosphincter is located on the ventral and lateral aspects (omega shaped) of the urethra. No clear, defined line could be identified between the transverse smooth muscle cranially and the striated muscle caudally. Rather, a gradual transition was noted in the middle third of the urethra, with intermingling fibers of both types of muscle (Colleselli et al, 1998). This area has been found to correspond to the area of continence region on fluorourodynamic studies performed on women who had undergone orthotopic reconstruction after cystectomy (Grossfeld, 1996). Branches off the pudendal nerve coursing beneath the levator muscle can be traced to the rhabdosphincter. Delicate fibers from the perineal portion of the pudendal nerve course underneath the urogenital diaphragm, entering the caudal portion of the urethra laterally. In a neuroanatomic study performed in male human cadaveric pelves, similar anatomic findings and innervation were described. Strasser and Bartsch (2000) described the male rhabdosphincter as an independent muscle unit that is not in direct contact with the fibers of the levator ani muscle. These dissections demonstrated that the male sphincter does not form a horizontal muscular ring around the membranous urethra. Rather, the male rhabdosphincter is a muscular coat situated ventral and lateral to the membranous urethra and prostate, the core of which is an omega-shaped loop that surrounds the membranous urethra. The innervation of the male rhabdosphincter was also found to originate from fine branches that arise off the pudendal nerve. These authors suggested that injury to either the rhabdosphincter or the pudendal innervation may impair the sphincter mechanism in men (Strasser and Bartsch, 2000). Attention to anatomic and surgical detail is important to optimize functional and clinical outcomes in patients undergoing orthotopic diversion after radical pelvic surgery. Minimal manipulation of the muscle fibers of the rhabdosphincter, fascial attachments, and corresponding innervation is essential in providing optimal urinary continence (Stein et al, 1997, 2001b; Colleselli et al, 1998; Stenzl et al, 1998; Strasser, 2000). The technique of an extended bilateral pelvic lymphadenectomy with radical cystoprostatectomy is well established (Stein and Skinner, 2004). Urethral preparation with preservation of the continence mechanism is critical when orthotopic diversion is anticipated. The continence mechanism in men may be maximized if dissection in the region of the pelvic floor and anterior urethra is minimized. Attention to surgical technique is important and is described in detail elsewhere (Stein et al, 2001b). Several fundamental key surgical issues in the preparation of the urethra in patients undergoing orthotopic diversion deserve special mention. Several methods can be performed to properly control the dorsal venous complex (DVC) (Stein et al, 2001b; Stein and Skinner, 2006). One may carefully pass an angled clamp beneath the dorsal venous complex, anterior to the urethra to pass a suture to ligate the complex. Alternatively, a suture on a long curved needle may be directly passed just anterior to the urethra, posterior to the venous complex, and tied as a simple or figure-of-eight stitch. A third alternative is to gather the complex in a long Allis clamp before passing the suture. The suture is best placed with the surgeon facing the head of the table and with the needle holder perpendicular to the patient. These latter maneuvers avoid passage of any instruments between the dorsal venous complex and the rhabdosphincter, which could potentially injure these structures and compromise the continence mechanism. Alternatively, especially in a patient with a broad, short DVC, the complex can simply be sharply divided and oversewn as needed to obtain adequate hemostasis. Absorbable suture should be used to avoid any risk of subsequent suture erosion into the urethral anastomosis. When orthotopic diversion is considered in female patients, several technical issues should be noted to optimize the continence mechanism (Stein et al, 2001b; Stein and Skinner, 2004). A standard female cystectomy includes removal of the uterus, cervix, and ovaries (anterior exenteration). However, in selected females with clinically lower-stage disease some authors have advocated preservation of the uterus and ovaries (Chang et al, 2002; Zippe et al, 2005). In either case, whenever possible the bladder is dissected completely off of the vagina, but a tumor on the posterior bladder wall may necessitate excision of a portion of the anterior vaginal wall. This does increase the risk of subsequent pouch-vagina fistula but is not an absolute contraindication to orthotopic reconstruction. However, a patient with a significant tumor at the bladder neck or with palpable extention into the vaginal wall is a poor candidate for neobladder and should undergo en bloc urethrectomy and cutaneous diversion. The issue of nerve-sparing cystectomy in women is controversial. Some authors have suggested that a sympathetic nerve-sparing cystectomy may be important in maintaining continence in women undergoing orthotopic diversion (Stenzl et al, 1995a; Hautmann, 1997; Turner et al, 1997; Bhatta et al, 2007). Others have routinely sacrificed the autonomic nerves coursing along the lateral aspect of the uterus and vagina and have successfully relied on the pudendal innervation of the rhabdosphincter complex for continence (Stein et al, 1997, 2004; Ali-El-Dein et al, 2002). In fact, the authors have observed good continence results in women undergoing complete bowel and urinary tract reconstruction after total pelvic exenteration with removal of all pelvic autonomic innervation. This, again, supports the concept that autonomic innervation is not critical to the continence mechanism.
Evolution of Orthotopic Urinary Diversion
Basic Principles of Continent Orthotopic Urinary Diversion
Patient Selection
Cancer-Related Factors
Risk of Urethral Recurrence in Men
Risk of Urethral Recurrence in Women
Locally Advanced Tumor Stage
Patient-Related Factors
Age
Renal Function
Manual Dexterity and Willingness to Do Self-Catheterization
Prior Pelvic Radiation
Prior Prostate Surgery or Bowel Resection
Continence Mechanism in Patients Undergoing Orthotopic Diversion
Surgical Techniques for Continence Preservation during Radical Cystectomy
Anterior Apical Dissection in the Male Patient
Urethral Dissection in the Female Patient
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Orthotopic Urinary Diversion
• Historically, urinary diversion has developed along three paths: a conduit form of diversion, continent cutaneous diversions, and most recently orthotopic diversion.
• The presence of carcinoma in situ, multifocal tumor, or extravesical disease should not preclude orthotopic diversion if the urethral margin is negative.
• Orthotopic lower urinary tract reconstruction can be performed after definitive, full-dose pelvic irradiation in carefully selected male and female patients.