Chapter 98A Orthotopic liver transplantation
Overview
Since the initial descriptions of orthotopic liver transplantation (OLT) in the 1960s, both the number of patients receiving transplants and the indications for the procedure have increased significantly. OLT represents the only treatment modality for many patients with a diverse spectrum of disease, with the predominant common factor being end-stage liver failure. It also has become an excellent option as curative therapy for early stage hepatocellular carcinoma (HCC). Advances in perioperative care of both donor and recipient, organ preservation methods, and surgical techniques have resulted in a 1-year overall survival of 88% for all recipients (Wolfe et al, 2010).
This chapter presents a broad overview of liver transplantation, including common criteria for recipient and donor selection (see Chapter 97A), standard operative approaches for donors and recipients (see Chapter 99), common postsurgical complications (see Chapter 100), and outcomes related to the underlying etiology of end-stage liver disease (ESLD). Specialized transplant techniques, such as split-liver and living-related donor liver transplantation, are described in Chapters 98B and 98C.
Patient Selection
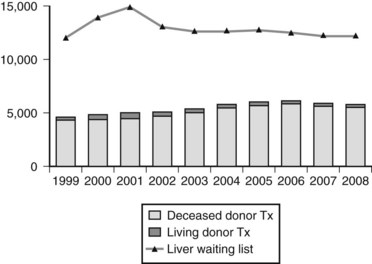
OLT represents the only curative treatment option for most patients with irreversible acute and chronic liver disease and cirrhosis, regardless of cause. Over the past 4 decades, 5-year patient survival after liver transplantation has increased from less than 50% to greater than 70% or more (Busuttil et al, 2005; Jain et al, 2000). The improvement in patient outcome has led to an expansion in the indications for transplantation and a concomitant increase in the number of patients referred to transplant centers (see Chapter 97A). The downside to this success is the persistent disparity between the number of recipients vying for a transplant and the number of donor organs available, a relatively constant supply (Fig. 98A.1).
The number of patients listed for OLT has increased sixfold since 1993, whereas the number of transplantations performed increased by only 45% during the same time. In 2008 in the United States, 15,807 patients were on the waiting list for a liver transplant, a decline from a peak of nearly 17,000 in 2002 (Wolfe et al, 2010); however, only 5817 of the patients on the waiting list received a liver allograft, and 90% of these recipients had a Model for End-Stage Liver Disease (MELD) score above 15 at transplantation (U.S. Scientific Registry of Transplant Recipients [USRTR], 2008).
Historically, the donor-to-recipient disparity leads to longer waiting times and worsening medical status with a peak waiting-list attrition rate of 187 per 1000 patient-years at risk in 1999. This rate declined by 15% and has remained steady over the last few years at 160 per 1000 patient-years at risk (Thuluvath et al, 2010). Because of the limited supply of donor organs, appropriate recipient and donor selection is paramount to improve resource use and long-term outcome.
Recipient Selection
Common indications for OLT (see Chapter 97A) include portal hypertension as manifested by variceal bleeding, ascites, encephalopathy, hyperbilirubinemia, hepatic synthetic dysfunction, and lifestyle limitations. More than 70% of liver transplantations are for noncholestatic liver disease, of which the most common etiologies are viral hepatitis (30%) and alcoholic cirrhosis (16%) (USRTR, 2008). Biliary atresia is the most common indication for liver transplantation in patients younger than 18 years of age (Busuttil et al, 2005; Goss et al, 1998). Nonalcholic steatohepatitis (NASH) is becoming an increasingly common cause of liver cirrhosis, as the prevalence of nonalcoholic fatty liver disease (NAFLD) increases; NAFLD now affects an estimated 10% of children (Schwimmer et al, 2006).
Few true, absolute contraindications to OLT exist that uniformly portend a poor patient outcome (Box 98A.1). Advanced cardiopulmonary disease, known extrahepatic malignancy, uncontrolled systemic sepsis from a source originating outside the liver, acquired immunodeficiency syndrome (AIDS), and ongoing or recent substance abuse are absolute contraindications. Many of the relative contraindications are conditions that are expected to improve after successful OLT. Examples include severe hemodynamic instability (e.g., shock) requiring multiple pharmacologic agents to maintain perfusion and severe hypoxia uncorrected by conventional intensive care measures in the context of hepatopulmonary syndrome. Other relative contraindications to OLT are extensive mesenteric venous thrombosis, morbid obesity, psychiatric disorders uncontrolled by conventional means, absence of a suitable social support network, and extremes of age (Jain et al, 2000; Loinaz et al, 2002; Rustgi et al, 2004).
Increasingly, advances in surgical technique and medical supportive care are overcoming obstacles and comorbid conditions formerly considered absolute contraindications to transplantation, including portal vein thrombosis (PVT), human immunodeficiency virus (HIV) infection, and advanced age. Patients who receive liver transplants now have higher rates of diabetes mellitus (DM), renal insufficiency, and morbid obesity (Thuluvath et al, 2010). Although pretransplantation insulin-dependent DM is not a contraindication to transplantation, evidence suggests that better risk stratification of these patients is warranted (Thuluvath, 2005). Likewise, patients with extreme body mass indexes (<18.5 or >40) might be at higher risk for posttransplantation complications (Dick et al, 2009). Morbid obesity is also associated with an increased risk of infectious complications and posttransplantation malignancy.
Although HIV infection has historically been considered a contraindication to OLT, the advent of highly active antiretroviral therapy (HAART) has turned HIV into a chronic condition, and patients are now living long enough to suffer the morbidity and mortality of other diseases, including ESLD. Therefore some patients with HIV may be considered for liver transplantation, if their CD4 T-cell count is greater than 200 and their HIV RNA viral load is less than 50 copies/µL within 12 months prior to transplantation (Di Benedetto et al, 2008). It should be noted that hepatitis C virus (HCV) coinfection with HIV predicts worse outcomes after OLT, with faster and higher rates of HCV recurrence (Di Benedetto et al, 2008).
The etiology of liver failure may be predictive of outcome after OLT, although the correlation between preoperative risk and graft survival sometimes varies. Jain and colleagues (2000) examined outcomes after OLT in 4000 consecutive patients treated at the University of Pittsburgh. Patients with metabolic or autoimmune liver disease experienced 10-year survival rates greater than 60%, whereas the survival rates for viral hepatitis or alcohol-induced cirrhosis were 40% to 50%, and patients transplanted for advanced hepatic malignancy had only a 22% survival at 10 years. Results for all patients were better in the most recent era. Earlier contradictory reports notwithstanding, recipient age probably does not influence transplantation results significantly (Gayowski et al, 1998; Ploeg et al, 1993; Totsuka et al, 2004). Thorough screening for medical comorbidities commonly found in older populations—such as lifestyle-limiting cardiopulmonary disease, systemic vascular disease, and chronic renal insufficiency—is crucial to successful OLT in patients older than 60 years.
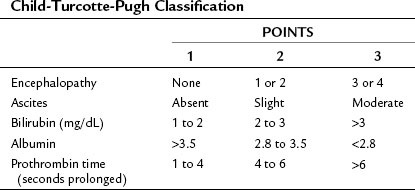
The recipient selection process has undergone extensive revisions to ensure equitable allocation of a scarce resource (cadaveric donor livers) while attempting to avoid futile transplantation. Before February 2002, recipients awaiting OLT were prioritized based on the Child-Turcotte-Pugh (CTP) scoring system (Table 98A.1), time on the waiting list, and patient location (e.g., intensive care unit). Waiting lists grew under this system, and it became increasingly clear that these parameters were not good measures of disease severity.
In 2000, the Department of Health and Human Services issued a guideline stating that the allocation of livers for transplantation should be based primarily on medical urgency (Freeman et al, 2002). The United Network of Organ Sharing (UNOS), which administers the Organ Procurement and Transplantation Network, commissioned several subcommittees to examine new methods for allocation. The result was the universal adoption of the MELD criteria (Table 98A.2) as a measure of the potential recipient’s necessity for OLT. Concurrent revision of the pediatric liver allocation process produced the Pediatric End-Stage Liver Disease (PELD) model, which is discussed further in Chapter 98C. Per a policy change in 2005, PELD is applied to potential transplant recipients younger than 12 years. Adolescents aged 12 to 17 years are stratified using the MELD system.
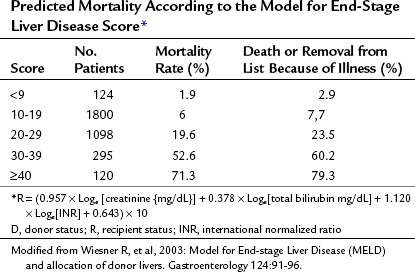
In contrast to its predecessor, the CTP score, MELD has been validated extensively as a predictor for 3-month mortality from chronic liver disease (Malinchoc et al, 2000; Wiesner et al, 2003a). It incorporates serum creatinine, bilirubin, and international normalized ratio (INR) as markers for risk of death; relies on objective and readily available blood tests; and practically eliminates time on the waiting list from consideration. Using MELD criteria, rates of death while awaiting OLT and removal from the waiting list for being too sick have decreased (Thuluvath et al, 2010). Median time to transplant in 2007 was 361 days.
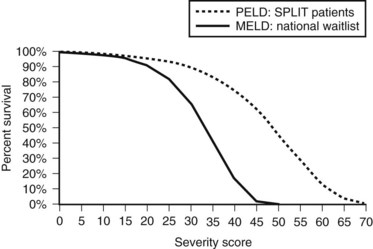
As shown in Figure 98A.2, the use of MELD criteria for predicting mortality from ESLD results in an appropriate correlation between severity score and actuarial survival at 3 months. Survival benefit analysis of liver transplant recipients stratified by MELD score demonstrates that the risks involved in transplantation are equivalent or less than the risks associated with remaining a transplant candidate on the waiting list with a MELD score over 15 (Merion et al, 2005). The MELD-based allocation process is dynamic and amenable to subtle changes to reflect an evolving understanding of the diverse pathology and physiology seen in patients treated with OLT.
Special considerations for certain subsets of patients with liver disease are ongoing. Early in the initial evaluation of the MELD criteria, it was recognized that patients with HCC and early cirrhosis should be prioritized lower on the waiting list, when they could potentially benefit most from transplantation (e.g., HCC at an early tumor stage; see Chapter 97D). Conceivably, patients with HCC but low MELD scores could develop progressive disease, which would eliminate OLT as a treatment option. Since the most recent revisions to MELD, patients with stage II HCC currently are allocated 22 MELD priority points. Criteria for selecting HCC patients for transplantation are discussed further in this chapter, including those with more advanced stage disease. Guidelines also exist for awarding priority points for other conditions, such as hepatopulmonary syndrome, portopulmonary hypertension, hepatic artery thrombosis after liver transplant, pediatric hepatoblastoma, inborn errors of metabolism, familial amyloidosis, and primary oxaluria; however, the process is highly variable by region (USRTR, 2008).
Since 2002, the number of patients on the waiting list with exception points for any condition has more than doubled, with close to 900 on the list in 2008 (Thuluvath et al, 2010). Increasingly, stage I and II hilar cholangiocarcinoma is becoming an acceptable indication for OLT, and uniform criteria for obtaining MELD exception points have been proposed (Gores et al, 2006; see Chapter 97E). Eligible patients must be treated according to a specific protocol that requires neoadjuvant chemoradiation, followed by a staging laparotomy to confirm absence of N1 disease (Rea et al, 2005).
Donor Selection
Increased demand for donor organs, as depicted in Figure 98A.1, has resulted in the concomitant increase in the use of less than ideal donors. The theoretic ideal donor is an otherwise healthy, hemodynamically stable young person who suffers an irreversible cerebral insult that results in brain death; this situation is rarely realized. Although marginal donors are being used more frequently, absolute contraindications remain, including known extracranial malignancy (except for basal or squamous cell cancer of the skin), overwhelming sepsis, hepatic cirrhosis, hepatic macrosteatosis greater than 60%, and HIV infection. Generally, hepatitis B virus (HBV) surface antigen and core antibody positivity in the donor also constitute absolute contraindications (Wachs et al, 1995), although many centers consider transplantation of core antibody-positive livers for HBV-positive recipients, if the graft seems otherwise suitable, and even into non-HBV recipients in selected circumstances. Advanced donor age may adversely affect graft survival, and in practice, donors older than 80 years usually are excluded from consideration.
Data that demonstrate differential effects on specific recipient groups, such as HCV-positive recipients, remain inconclusive (Doyle et al, 2008; Lake et al, 2005; Russo et al, 2004). Although liver allografts with 30% to 60% steatosis are utilized in select groups of recipients, it should be recognized that greater than 60% steatosis significantly increases the risk of primary nonfunction (Urena et al, 1999). A donor risk index (DRI) has been developed to help quantify and stratify allografts by predicted failure risk (Feng et al, 2006).
As a result of the shortage of cadaveric donors, the use of living donors has increased in the United States, from 1996 with a peak in 2001; it has since declined steadily, and LDLTs now represent only 4% of liver transplantations performed (see Chapter 98B). A shift in demographics has also been seen in recipients of LDLT: patients age 50 to 64 years represent the largest group (USRTR, 2008). LDLT is appealing, because it allows elective transplantation after optimizing the health of the recipient, reduces cold ischemic times, and potentially shortens waiting times. The disadvantages include morbidity and rare mortality in the donor population (Brown et al, 2003). The most common practice in adults is transplantation of the right hepatic lobe. Despite initial data indicating worse outcomes in LDLT, a trend has been seen toward improved graft survival and overall survival in the United States in the last 10 years (Thuluvath et al, 2010). Although LDLT from an adult to a child has become well accepted and standardized, adult-adult LDLT continues to evolve, and the place of this approach as part of the overall picture of liver transplant options is not fully defined.
DCD livers are increasingly being used and now represent 5% of all liver-only transplants (USRTR, 2008). Compared with historic results with brain-dead donors, outcomes using DCD allografts are less optimal, with 1- and 3-year graft survivals of 70% and 60%, 10-year graft survival of 44% for all patients, and a primary nonfunction rate of almost 12% (Abt et al, 2004; Bernat et al, 2006; D’Alessandro et al, 2000; de Vera et al, 2009; Mateo et al, 2006; Thuluvath et al, 2010). The graft survival rate improves when donor warm ischemia time is less than 30 minutes and cold ischemia time is less than 10 hours (Mateo et al, 2006); however, DCD grafts are associated with an increased risk of nonanastomotic intrahepatic biliary strictures (ischemic cholangiopathy) and posttransplantation hepatic artery stenosis (Chan et al, 2008; Pine et al, 2009). Given the risks inherent in DCD allografts, they should be used cautiously in select groups of recipients.
Operative Techniques
The first published description of human liver transplantation was by Starzl and colleagues in 1963 at the University of Colorado. In this seminal paper, the dismal outcomes of three OLT recipients were described, including one intraoperative death from uncorrectable coagulopathy and two survivors of 7 and 22 days. In addition to the pioneering conceptual framework and implementation of liver transplantation, several advanced techniques are presented in this reference, including the use of grafts from non–heart-beating donors, venovenous bypass in the recipients, choledochocholedochostomy, and coagulation monitoring using thromboelastography (TEG). Many of these concepts remain or have reentered the realm of liver transplantation more than 40 years after their initial description. Based largely on the initial body of work by Starzl and colleagues, this section describes the surgical procedures commonly used at Washington University in St. Louis during OLT (see Chapter 99).
Donor Hepatectomy
Management of a cadaveric organ donor begins preoperatively, immediately after identification of a candidate and after evaluation by trained transplant coordinators. After brain death, severe physiologic derangements can occur, and physiologic instability increases in proportion to the length of time between declaration of death and organ procurement (Nygaard et al, 1990). The progression from brain death to somatic death results in the loss of 10% to 20% of potential donors (Wood et al, 2004).
Complications that commonly occur in a brain-dead donor include hypotension, the requirement for multiple transfusions, disseminated intravascular coagulopathy (DIC), diabetes insipidus (DI), pulmonary edema and hypoxia, acidosis, and arrhythmias and cardiac arrest. Intravascular volume repletion to normovolemia is the cornerstone of management; however, vasopressors or inotropic agents often are necessary to achieve an adequate perfusion pressure. The use of low-dose arginine vasopressin allows a reduction in the dosing of α-adrenergic agents, which may impair end-organ perfusion (Pennefather et al, 1995). Directed therapy using a pulmonary artery catheter can improve outcome in patients with brain death–induced or traumatic cardiac dysfunction (Wheeldon et al, 1995). A thorough review of the medical management of potential organ donors can be found elsewhere (Wood et al, 2004).
Surgical techniques for procuring abdominal organs from brain-dead, heart-beating donors have been described previously (Farmer et al, 2000; Merkel et al, 1972; Starzl et al, 1984, 1987). A midline incision from the suprasternal notch to the pubis is performed, followed by sternotomy and entry into the peritoneum. The abdomen is inspected for any evidence of malignancy or gross gastrointestinal ischemia, which would preclude transplantation. Procurement proceeds in several phases: warm dissection and cannulation, exsanguination with cold perfusion and organ removal, and back-table dissection and organ preparation.
Procurement in a DCD donor requires a slight modification (Bernat et al, 2006; D’Alessandro et al, 2000). In the controlled setting, the donor is brought to the operating room, and support is withdrawn. Heparin is administered to reduce risk of thrombus formation in the graft (Bernat et al, 2006). Apnea and cessation of circulation ensue after a variable amount of time, at which point of death is declared. It is important to note that 10% of potential donors do not die within 2 hours of withdrawal of support; these patients are not candidates for subsequent organ donation and are transferred back to the intensive care unit (ICU) and are allowed to die (Cooper et al, 2004). Circulation is unlikely to resume after 2 minutes of complete cessation; a minimum waiting period of 2 minutes is required, and a 5-minute interval between asystole; pronunciation of death prior to further intervention is strongly encouraged (Bernat et al, 2006).
The goal now becomes rapid reperfusion of the organs for procurement with cold preservation solution; this usually is accomplished via a quick midline laparotomy and cannulation of the aorta. Alternatively, some centers use cannulae placed before death in the femoral artery and vein. Less than 30 minutes of donor warm ischemia time is generally considered acceptable (Bernat et al, 2006). Hepatectomy is performed as in a standard brain-dead donor, commonly followed by a back-table flush. Cadaveric split-liver and living-related liver transplantation techniques are addressed in Chapters 98B and 98C.
Recipient Hepatectomy
Next, the portal vein is skeletonized proximally to just above the confluence of the splenic and superior mesenteric vein. At this point, further dissection is influenced by the use of temporary portocaval shunting or venovenous bypass; both techniques allow decompression of the splanchnic circulation, which reduces bowel edema during the anhepatic phase. When venovenous bypass is used, the portal vein is cannulated, and bypass is instituted as previously described (Shaw et al, 1984). A potential disadvantage of venovenous bypass is the added complexity and potential complications associated with the bypass process (e.g., thrombosis of the bypass circuit). We prefer portocaval shunting with an end-to-side anastomosis between the divided portal vein and infrahepatic IVC. The shunt is kept in place until the suprahepatic caval anastomosis is completed.
Temporary portocaval shunting in conjunction with the piggyback technique may improve intraoperative hemodynamic stability and renal function and may reduce the transfusion requirement (Arzu et al, 2008; Davila et al, 2008; Figueras et al, 2001). If neither venovenous bypass nor temporary portocaval shunting is used, the portal vein simply can be clamped proximally, ligated in the hilum, and divided; however, this method may be associated with a severe reduction in venous return, up to a 50% decrease in arterial blood pressure, mesenteric venous hypertension and associated organ failure, and potential increased hemodynamic instability resulting in intraoperative mortality (Hoffmann et al, 2009).
With the portal vein bypassed or clamped, exposure for the infrahepatic dissection and vascular control of the IVC is easily obtained. The piggyback technique leaves the recipient retrohepatic IVC intact and requires ligation and division of all retrohepatic caval branches (Tzakis et al, 1989). The advantage of this approach is that native caval flow is maintained, and venovenous bypass is not required; the disadvantage is that division of retrohepatic caval branches can be tedious and time consuming. The donor suprahepatic IVC is anastomosed to the confluence of the right, middle, and left hepatic veins, which are joined in a common cuff. Vascular control is achieved by clamping the hepatic veins at their point of entry into the vena cava.
Alternatively, if a bicaval technique is used, the recipient’s native retrohepatic IVC is removed with the native liver. The retrohepatic IVC is mobilized out of the retroperitoneum from the left side. The right triangular ligament is taken down, and the retrohepatic IVC is dissected from the right side. The adrenal vein is ligated in the traditional bicaval approach; this dissection frees up the retrohepatic IVC above the hepatic veins to allow application of infrahepatic and suprahepatic IVC clamps. The recipient liver is sharply excised with care taken to leave cuffs of IVC above and below the liver. This technique allows for true orthotopic placement of the donor graft. Retrospective analysis of the bicaval and piggyback techniques suggest that safety and outcomes are comparable (Nishida et al, 2006).
Recipient Implantation
OLT requires three or four vascular anastomoses in the following order: 1) suprahepatic IVC; 2) infrahepatic IVC, if a bicaval technique is used; 3) portal vein; and 4) hepatic artery. Alternatively, the use of the piggyback technique allows just a single caval (end-to-side) anastomosis, with simple ligation of the donor infrahepatic IVC; this results in a reduction in the duration of the anhepatic phase (Hosein Shokouh-Amiri et al, 2000). When the liver is fully reperfused, reconstruction of the biliary tract begins.
Adequate cuffs of suprahepatic and infrahepatic IVC are essential for reconstruction (Starzl et al, 1979). Anastomoses of these cuffs require reconstruction of the posterior walls from within the lumen using a running 3-0 polypropylene suture. The anterior layer is sutured externally using either an interrupted or a continuous technique. Another advantage of the piggyback technique is the use of a side-biting vascular clamp on the IVC at the level of ostia of the hepatic veins. Although this clamp may impair venous return to the heart to some degree during a clamp time of 15 to 30 minutes, it generally produces greater hemodynamic stability during the anhepatic phase than does complete caval occlusion, which requires a bicaval procedure (Moreno-Gonzalez et al, 2003). After the hepatic donor vena cava has been anastomosed to the recipient IVC, the side-biting clamp can be moved to the graft side of the anastomosis, restoring complete venous return, while the remaining vascular connections are performed.
The final vascular anastomosis is arterial. The key principle of hepatic arterialization is to ensure pulsatile inflow through a large caliber vessel over a short length (Farmer et al, 2000). The technique involves the use of a fine (7-0) monofilament suture in a running or interrupted fashion. The vessel ends are frequently spatulated and are sewn from the outside and rotated to achieve the most precise anastomosis. The presence of aberrant hepatic arterial anatomy is encountered in 10% to 30% of grafts, and preservation of these vessels is essential for successful engraftment. Reconstruction of the aberrant vessels to obtain a single inflow vessel is imperative and takes place during the back table preparation of the graft as described earlier.
Recipient inflow is obtained from a branch off the celiac trunk, usually the proper or common hepatic artery. When adequate arterial inflow cannot be obtained by this artery, the use of an arterial conduit is recommended. Inflow originating from the infrarenal and supraceliac aorta has been described; both methods provide excellent inflow, and the choice is based on technical considerations and surgeon preference. The best choice of conduit is usually the donor iliac vessels. When donor vessels are unavailable or inadequate, prosthetic conduits can be used, typically polytetrafluoroethylene (PTFE). In the perioperative period, aortic conduits are associated with increased operating time, greater transfusion requirements, and respiratory and renal failure (Nikitin et al, 2008). In addition, several cases of internal hernias with small bowel volvulus around the intraperitoneal conduit have been reported (Nishida et al, 2002).
Prior to anastomosis, both the donor and recipient bile ducts should be trimmed sharply to remove devitalized tissues, and brisk bleeding should be evident from the cut ends. The recipient bile duct should be cleared of sludge and stones. Accommodation for size mismatch with ductoplasty of the larger duct or spatulation of both ducts has been described (Buczkowski et al, 2007; Nissen & Klein, 2009). The choledochocholedochostomy and choledochojejunostomy anastomoses are accomplished with the use of fine absorbable monofilament suture in a single-layer closure.
Whether to stent the biliary anastomosis during OLT is subject to debate (Barkun et al, 2003; Bawa et al, 1998; Johnson et al, 2000). Proponents believe that decompression of the biliary tree reduces the rate of clinically significant leaks, whereas others point to data that suggest a higher rate of biliary stricture from internal stents. At our institution, biliary stenting is performed selectively based on individual case circumstances and surgeon preferences.
Complications
Primary Nonfunction
Primary nonfunction is defined as early graft failure after OLT in the absence of identified technical complications. Clinical presentation varies, but patients typically are seen with alterations in mental status, diminished bile production, coagulopathy, markedly elevated transaminases, and metabolic acidosis. Multiorgan failure ensues, with oliguria and hypoxia occurring frequently. The reported rate of primary nonfunction varies between 1% to 7% of all OLTs (Jain et al, 2000; Johnson et al, 2007; Kamath et al, 1991; Kemmer et al, 2007; Taner et al, 2008; Totsuka et al, 2004). Retransplantation is the treatment of choice and typically is required within 72 hours. Some authors also report the distinctly separate category of initial poor function, in which allografts might have a chance for functional recovery; however, no consensus for a clear definition exists.
The etiology of primary nonfunction is unclear but is likely multifactorial. Factors commonly reported to be associated with nonfunction include prolonged cold and warm ischemic times, donation after cardiac death, intraoperative systemic hypotension (mean arterial pressure <40 mm Hg), severity of donor steatosis, advanced donor age, and recipient factors such as portal vein thrombosis (PVT), renal failure, dependence on life support, and status 1 listing (de Vera et al, 2009; Fernandez-Merino et al, 2003; Johnson et al, 2007; Marsman et al, 1996; Nair et al, 2002; Ploeg et al, 1993; Reich et al, 2003; Sharma et al, 2010; Strasberg et al, 1994). Although it has been proven that severe macrosteatosis (>60%) increases risk of graft failure, the influence of moderate steatosis (30% to 60%) remains unclear (Yoo et al, 2003a), and such allografts can be used with careful selection (Doyle et al, 2010).
Donor liver biopsy to quantify graft steatosis should be performed before implantation of any graft that appears marginal (D’Alessandro et al, 1991). As would be expected, the likelihood of primary nonfunction is increased in the presence of multiple risk factors (e.g., steatotic graft with extended total ischemic time) and in allografts with several marginal donor criteria (Pokorny et al, 2005; Salizzoni et al, 2003).
Hepatic Artery Thrombosis
Hepatic artery thrombosis (HAT) can be broadly divided into early (acute) and late (delayed) clinical presentation, with different etiologies, patient manifestations, and treatments. Although there is no formal consensus, early HAT is typically defined as occurring within the first 1 to 2 months after OLT and has a mean incidence of 2.9% in adult OLT patients, with a significantly higher incidence of 8% to 10% in the pediatric population (Bekker et al, 2009; Farmer et al, 2007). Early HAT carries a high risk of graft loss and death—53% and 33%, respectively (Bekker et al, 2009).
Technical, donor, and recipient factors contribute to an increased risk of HAT (Bekker et al, 2009; Del Gaudio et al, 2005; Duffy et al, 2009; Jurim et al, 1995; Soin et al, 1996; Vivarelli et al, 2004). Donor factors may include small-caliber vessels, aberrant anatomy that requires complex arterial reconstruction, use of aortic conduits, or cytomegalovirus (CMV) seropositivity donor-recipient mismatch. Variable recipient anatomy (e.g., replaced hepatic artery from superior mesenteric artery [SMA]) also influences risk of HAT.
Some reports suggest that endovascular procedures—intraarterial thrombolysis, percutaneous transluminal angioplasty, and endoluminal stenting—can be successful in hepatic arterial revascularization (Singhal et al, 2010); however, a majority of patients eventually require retransplantation (Bekker et al, 2009; Duffy et al, 2009). Because of the severity of this complication, some authors advocate the routine use of postoperative duplex US.
Late HAT is typically more indolent, frequently because a previously undiagnosed stricture allows collateral circulation to develop. Clinical presentation may include fever secondary to perihepatic abscess or biliary leak, biliary strictures, or cholangitis, although in some cases patients may be asymptomatic with no clinical consequences (Gunsar et al, 2003). Contributing factors are active tobacco abuse; coagulation abnormalities, such as factor V Leiden; cerebrovascular accident as donor cause of death; donor death at an age greater than 50 years; recipient CMV positivity; and use of donor iliac interposition graft (Del Gaudio et al, 2005; Gunsar et al, 2003; Pascual et al, 1997; Pungpapong et al, 2002; Stewart et al, 2009; Vivarelli et al, 2004).
Treatment may be attempted with endoscopic or percutaneous biliary decompression, stenting, or even systemic anticoagulation. Retransplantation is required less for late HAT than for early HAT. Because postoperative antiplatelet therapy may reduce the rate of late HAT in high-risk patients (Vivarelli et al, 2007), it is our practice to prescribe daily aspirin (81 mg) for all patients.
Hepatic Artery Stenosis
Hepatic artery stenosis (HAS) without thrombosis is also a recognized complication of liver transplantation, with an incidence of 4% to 11% (da Silva et al, 2008). DCD allografts have been implicated as a risk factor (Pine et al, 2009). Initial presentation typically includes a mild increase in aminotransferase levels with or without associated graft dysfunction or biliary complications. Doppler US demonstrates increased resistance in hepatic arterial flow and is often diagnostic, but confirmation by arteriography is the gold standard. Therapeutic options include angioplasty with or without stenting. Restenosis can occur in up to one third of patients within 1 year after stenting (Ueno et al, 2006).
Portal Vein Thrombosis
PVT occurs less frequently than HAT after OLT, with an incidence less than 2% in adult recipients and 10% in pediatric recipients (Duffy et al, 2009; Lerut et al, 1987; Millis et al, 1996), although PVT adversely affects overall survival after OLT (Duffy et al, 2009). Low portal flow, small-diameter veins (<5 mm), preexisting PVT in the recipient, donor-recipient vessel size mismatch, and the use of vascular grafts for reconstruction are known risk factors for developing PVT (Cheng et al, 2004).
PVT is typically symptomatic, and patients can present with acute hepatic failure, as with HAT, or with the sequelae of portal hypertension, such as increasing ascites, splenomegaly, and variceal hemorrhage (Duffy et al, 2009). Diagnosis is made using duplex US or contrast-enhanced CT portal venography.
Depending on the timeliness of diagnosis and the acuity of the patient, several treatment options exist. In patients with fulminant hepatic failure from PVT, reexploration and attempted portal revascularization are performed. These patients sometimes require retransplantation, particularly in the rare setting of combined PVT and HAT (total absence of hepatic inflow). The use of a portocaval shunt to augment flow through the reconstructed portal vein has been described (Bakthavatsalam et al, 2001), as has the use of transjugular intrahepatic portocaval shunt (TIPS) in conjunction with thrombolytics (Ciccarelli et al, 2001
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree