Fig. 3.1
Development of a hematoma anterior to the stomach following a laparoscopic sleeve gastrectomy
The second location of postoperative bleeding is intraluminal. Again, it occurs most commonly along staple lines (e.g., gastroenterostomy, gastric staple line, or enteroenterostomy). Surgeons have employed the use of staple-line reinforcements and topical hemostatic agents/sealants for prophylaxis against such risk of bleeding. Dapri et al. showed the benefit of buttressing materials in reducing the incidence of postoperative bleeding without any statistically significant effect on the occurrence of leaks or operative time [43]. A systematic review of 30 articles, performed by Knapps et al., demonstrated a lack of statistical difference for staple-line leak, bleeding, and other major complications in laparoscopic sleeve gastrectomy with or without staple-line reinforcement [44]. Thrombin and other newer hemostatic-sealant agents (Tachosil®) have shown promise in reducing postoperative bleeding in some case series but major randomized controlled studies are lacking [45, 46]. Thus, the benefit of these agents in preventing episodes of hemorrhage is a matter of great debate in the surgical literature.
Another uncommon, but possible, source of bleeding is Mallory-Weiss tear resulting from severe vomiting or retching. Preoperative education regarding eating choices and a graduated process of dietary advancement should help to reduce this occurrence.
Although the clinical presentation of acute bleeding with a drop in hemoglobin/hematocrit, diaphoresis, tachycardia, hypotension, abdominal distention, hematemesis, and melena is instrumental in making the diagnosis, identification and the corresponding control of the site of bleeding can be a challenge. Patients with early postoperative bleeding experience a significantly longer hospital stay (4.8 vs. 3.0 days, p < 0.0001) and higher mortality rate (7.1 % vs. 0.9 %, p < 0.01) compared with those without an early bleed [47].
The initial management of acute postoperative hemorrhage in this patient population does not differ from any other upper gastrointestinal bleed in a non-bariatric surgical patient. It includes adequate resuscitation, close monitoring of vital signs, serial blood counts, and discontinuation/reversal of anticoagulation. Blood product transfusion may be initiated when indicated. A majority of bleeding episodes will resolve without further surgical intervention. Patients with ongoing intraluminal bleeding along with high transfusion requirements will need further endoscopic exploration. It may reveal the site of bleeding on the inner aspect of the staple line, which can then be controlled by adrenaline injection, electrocoagulation, or endoclips. Hemodynamic instability and refractory bleeding, from the gastric remnant or other sites inaccessible to endoscopy or within the peritoneal cavity, can require surgical revisions. The operative goals are to evacuate the majority of the clots, attempt to identify, and control the site of hemorrhage if it is readily apparent or to oversew all staple lines if the patient is hemodynamically unstable and does not have an obvious bleeding source.
3.6.4 Nutritional Deficiencies
Anemia is the most common complication after a bariatric procedure and is estimated to occur in 20–49 % of patients. This may result from acute blood loss or secondary to deficiencies of iron, vitamin B12, or folate. Bariatric patients may also present with deficiencies in magnesium, calcium, zinc, copper, vitamin D, thiamine, and vitamin A. However, a vast majority of these deficiencies do not manifest in the postoperative period as an early complication except thiamine, which can have severe neurological consequences [48].
Thiamine deficiency has been reported to occur in up to 29 % of patients. It can present within the first 6 weeks following the bariatric intervention. A high degree of suspicion and clinical alertness is required for its prompt diagnosis and adequate treatment should be instituted immediately. Vomiting after a bariatric procedure is the principal risk factor for development of thiamine deficiency in combination with poor food and supplement intake. Acute symptomatic thiamine deficiency may be precipitated by administration of intravenous glucose, which leads to interruption of the citric acid cycle and lactic acidosis. The deficiency may manifest as Wernicke’s encephalopathy (ophthalmoplegia, nystagmus, ataxia, and mental status changes) or acute polyradiculoneuropathy (Guillain-Barre syndrome). If not recognized and treated, Wernicke’s encephalopathy can progress to death or chronic neurological impairment known as Korsakoff’s syndrome. The authors recommend prophylactic administration of intravenous thiamine 100 mg before starting intravenous fluids in patients at risk. Multivitamin supplementation should be carefully selected to include at least 3 mg of thiamine for prophylaxis. In patients demonstrating symptomatic deficiency, daily administration of 100–500 mg intravenous thiamine is recommended [49].
3.6.5 Anastomotic or Staple-Line Leak
Anastomotic or staple-line leak is one of the most dreaded complications of a bariatric procedure. It is considered as one of the strongest independent risk factors for postoperative mortality. The incidence of leaks, across different bariatric procedures, ranges from 1 to 5 %, depending on the series and the patient characteristics [50]. Revisional surgeries are associated with a higher rate of leaks and/or fistulas. It may present either early within the first 7 days after surgery or late, at a week or more after surgery. Early diagnosis of such a leak is critical to avoid progression of adverse outcomes of peritonitis, which include systemic inflammatory response, sepsis, multi-organ failure, and lastly death. The clinical presentation of these patients is identical irrespective of the procedure. Findings like tachycardia, pyrexia, tachypnea, abdominal pain, oliguria, nausea, and vomiting and purulent drain output are the harbingers of a leak. It has been demonstrated that a persistent heart rate in excess of 120 beats per minute is a good indicator of an anastomotic/staple-line leak. Kolakowski et al. further reported in a study that the triad of tachycardia, tachypnea, and fever was 58 % sensitive and 99 % specific for detection of anastomotic leaks [51].
These findings can then be supported with either an upper gastrointestinal contrast study or a computerized tomography of the abdomen with water-soluble oral contrast (e.g., Isovue) to confirm the diagnosis. Sensitivity of upper GI contrast studies varies among reports between 22 and 75 % (Fig. 3.2). The interpretation of computerized tomography of the abdomen is user dependent and fails to demonstrate a high level of sensitivity in detecting early postoperative leaks in this patient population. When upper GI and CT are combined, up to one-third of patients will have both studies interpreted as normal despite the presence of a leak [52]. Lastly, surgical exploration is the most definitive assessment of the possibility of a leak with the highest sensitivity, specificity, and diagnostic accuracy. It should be implemented in the presence of negative imaging studies when there remains a high suspicion of a leak. Despite the invasiveness of re-exploration, it is a much safer intervention in view of the severe consequences of a delayed diagnosis of this complication.
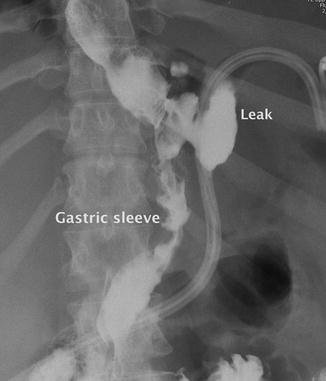

Fig. 3.2
Upper gastrointestinal study showing extraluminal contrast from the proximal staple line of a gastric sleeve
Adjustable gastric band may occasionally present with a “leak,” which is actually due to esophageal or gastric cardiac perforation due to blind tunneling in the retro-gastric fat during placement of the device. The most common location of leakage after a sleeve gastrectomy is at the proximal end of the staple line near the gastroesophageal junction followed by the site of intersection of consecutive staple lines. The gastrojejunostomy is considered as the high-risk anastomosis for a leak in a Roux-en-Y gastric bypass surgery.
Meticulous dissection, good surgical technique with gentle handling of tissues, and avoidance of tissue injury and ischemia are essential in preventing the occurrence and thereby avoiding the need to manage anastomotic/staple-line leaks. Most surgeons, including the authors, test the integrity of this anastomosis intraoperatively by insufflation of air via an orogastric tube, instillation of methylene blue through the same route, or flexible gastroscopy while keeping the anastomosis submerged in saline. Intraoperative gastroscopy is popular as it may also help in control of staple-line bleeds along with prompt evaluation of integrity of the anastomosis [53, 54]. Some authors advocate the use of fibrin sealant around the anastomosis to achieve better hemostasis around the suture line and prevent or decrease the incidence of anastomotic leaks [55].
When identified early in the postoperative course in a patient with unstable hemodynamics and/or florid sepsis, a return trip to the operating room is mandated for the management of leaks. A laparoscopic or open approach may be adopted based on the skill and expertise of the surgeon and associated patient factors. Control of the leak with possible repair, copious lavage of the peritoneal cavity, and placement of closed suction drains in the area of the leak and other dependent spaces are the mainstays of its operative management. On occasions when surgical repair of these leaks are not possible (e.g., patients with delayed presentation and associated inflammatory reaction and friable tissue), lavage with wide drainage alone is considered safe. With a potential for prolonged limited oral intake after operative drainage, one should consider placement of a feeding gastrostomy in the gastric remnant of patients post- Roux-en-Y gastric bypass or a feeding jejunostomy in SG and DS patients. A naso-enteral feeding tube can also be considered for enteral nutrition to aid in the healing of the leak [56].
Placement of an intraluminal endoprosthesis may be considered to manage proximal leaks from gastric sleeves . These allow adequate enteral diversion to aid in optimal healing when left in situ over 4–6 weeks [57]. However, the risk of migration and erosion of enteric stents appear to overshadow the benefits. Occlusion of the leak by injection of fibrin glue also shows promise. Management of leaks in patients who are not amenable to placement of stents with endoclips and Over-the-Scope Clip (OTSC) (Ovesco Endoscopy, Tübingen, Germany) has also been reported and these may be employed based on the expertise of the surgeon or gastroenterologist [58, 59].
If the patient is hemodynamically stable and does not demonstrate any signs of sepsis, non-operative management with fluid resuscitation, intravenous antibiotics, and bowel rest may be considered along with placement of percutaneous drains in the intra-abdominal collections [56].
3.7 Acute Complications Specific to Adjustable Gastric Band
Laparoscopic adjustable gastric banding is a safe bariatric procedure. However, these patients can present with unique complications that must be recognized and managed appropriately to achieve good outcomes. The major early complications include band slippage , band erosion , stomal obstruction secondary to a food bolus, malposition of the band, port infection, and port or tubing malfunction.
3.7.1 Band Slippage
Slippage of an adjustable gastric band may be defined as a cephalad prolapse of the body of the stomach through the band or caudal movement of the band. Its reported incidence is between 1 and 20 % across studies over the years. The normal location of the band is at the angle of His. When slippage of the band occurs, complete stomal obstruction of the stomach can be precipitated secondary to proximal protrusion of a larger cross-sectional area of gastric body through the narrow diameter of the band. Placement of gastro-gastric tunnel stitches, usually two or more around the gastric band , is considered important for the prevention of band slippage. Many authors advocate an additional gastropexy stitch between the fundus of the stomach and the left crus of the diaphragm (Birmingham stitch) to prevent this complication [60]. Gastric band slip may be classified into five types. Type I prolapse involves upward migration of the anterior wall of the stomach through the band likely due to improper anterior fixation and disruption of the fixation sutures. Type II prolapse involves herniation of the posterior wall of the stomach through the band due to improper surgical technique. The pars flaccida technique for placement of the band is considered superior to the perigastric technique in minimizing the occurrence of this complication. Type III prolapse is defined as dilation of the proximal stomach pouch without any signs of obstruction or change in the angle of the band. It may be associated with dilation of the lower esophagus. It is caused by elevated distal pressure secondary to band over-inflation or due to overeating over a period of time. Type IV prolapse is an immediate postoperative event due to lower placement of the band on the stomach. Type V prolapse comprises necrosis of the herniated stomach wall as a result of progression of types I and II prolapse. Types I, II, IV, and V present as an acute complication and may mandate urgent/emergent surgical intervention for removal or repositioning of the band based on the presenting signs and symptoms. Type III is a chronic complication, which is managed non-operatively with band deflation, food-portion control, and observation. Surgical treatment may be necessary if conservative management fails to reduce the size of the proximal stomach [61].
The initial presentation of a patient with band slippage includes persistent abdominal pain, dysphagia, vomiting, regurgitation, and food intolerance, which eventually may progress to gastric necrosis with perforation, upper gastrointestinal bleeding, and aspiration pneumonia. The radiological diagnosis is based on the orientation of the band on plain abdominal X-ray and an enlarged gastric pouch in an upper gastrointestinal contrast study or a computerized tomography of the abdomen with oral contrast (Fig. 3.3).
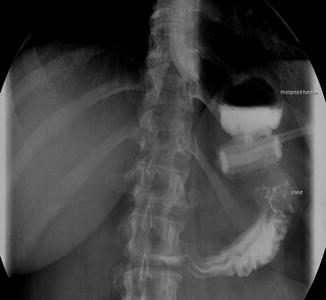

Fig. 3.3
Upper gastrointestinal study showing prolapse of the fundus of stomach secondary to slippage of an adjustable gastric band
The first step in the management of this acute complication is complete band deflation by accessing the subcutaneous port with a Huber needle under strict aseptic precautions. Patients may be offered laparoscopic repositioning of the gastric band if conservative management fails to control symptoms. If reduction of substantial prolapse is not feasible or there is evidence of associated intra-abdominal infection, laparoscopic removal of the gastric band should be performed [62].
3.7.2 Port-Site Infection
Early port-site infections are identified usually within the immediate postoperative period and present frequently as cellulitis. Use of perioperative antibiotics may help in reducing the incidence of this infection. If the infection involves an underlying abscess at the location of the port or failure of antibiotic treatment of the overlying cellulitis, the port should be removed. The proximal end of the tubing may be dropped within the peritoneal cavity for recovery at a later date following resolution of the infection. The possibility of band erosion leading to bacterial seeding of the tubing and access port should be considered and may be ruled out with upper endoscopy.
3.7.3 Port or Tubing Malfunction
Port or tubing malfunction may occur as a result of damage of the port septum or the tubing or as a result of inversion or dislodgement of the port. Damage to the port or to the tubing will result in slow leak of the injected fluid volume and manifest as a feeling of loss of restriction over a period of time after band inflation. This condition can be diagnosed by injecting contrast into the port under fluoroscopy, which will identify the site of leakage of contrast. Damage to the intra-abdominal tubing or the band can also be diagnosed during laparoscopy by injecting methylene blue dye intraoperatively in the port. Based on the site of leakage, surgical treatment may involve replacement of the port, tubing, or the band.
Port inversion may present with difficulty in accessing the port. This can be identified by abdominal radiography. Local exploration of the port site and fixation of the port to the underlying fascia will solve this problem. Surgeons have employed multiple methods of port fixation to avoid this problem including suturing to mesh and tacking the mesh to fascia. The recent addition of automatic fixation devices has greatly facilitated this process and has all but eliminated this problem.
3.7.4 Malposition of Gastric Band
Malposition or misplacement of gastric bands is a very rare but recognized early complication of gastric band surgery. It presents with symptoms of dysphagia and dyspepsia without any significant associated weight loss. This results due to erroneous placement of the band in the pericardial or retro-gastric fat pad. Misplacement of the band can be identified with a radiologic contrast study where contrast will be found to flow outside the circumference of the band [63] (Fig. 3.4). With diligent identification of anatomical landmarks , this issue can be avoided.
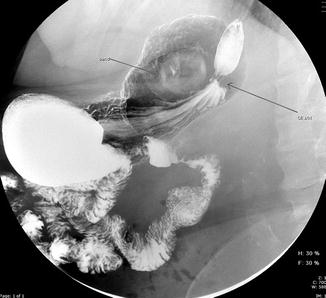

Fig. 3.4
Upper gastrointestinal study showing misplacement of an adjustable gastric band. The transit of contrast occurs outside the gastric band
3.8 Acute Complications Specific to Sleeve Gastrectomy
Laparoscopic sleeve gastrectomy is a relatively new and effective surgical option for the management of morbid obesity. Initially conceived as a bridging operation in high-risk patients before biliopancreatic diversion or Roux-en-Y gastric bypass, it has established itself as an independent procedure with great potential. Early major complications include hemorrhage, staple-line leak, mid-gastric stricture , and porto-mesenteric venous thrombosis.
3.8.1 Mid-Gastric Stricture
This is a potential complication occurring in <1 % of patients after a laparoscopic sleeve gastrectomy. It can present acutely after surgery secondary to tissue edema or more commonly in a delayed fashion. It usually results from close proximity of the staple line at the level of the incisura angularis, resulting in an hourglass appearance of the stomach. Further inflammation and scarring at this site lead to formation of the stricture. These patients are at a higher risk for leaks at the proximal staple line due to the presence of narrow diameter and concomitant higher pressure at the level of the incisura. This can be avoided by placing a 34–40 Fr bougie and maintaining an appropriate distance away from the incisura while firing the first couple of stapler loads. The usual presenting symptoms are nausea, vomiting, dysphagia, and food intolerance. An upper gastrointestinal contrast study or endoscopy is diagnostic for this condition.
During its acute presentation, the treatment is usually non-operative with complete bowel rest and intravenous hydration. Symptoms due to mid-gastric stenosis may be caused by postoperative edema and resolve spontaneously in the absence of other pathologies like leak or abscesses. Endoscopic or radiological dilation may be indicated in the event of failure of expectant management. Multiple successive interventions may be required to treat the condition and ameliorate the symptoms [64]. The above may not be effective in patients with long segment stenosis when surgical therapy may be mandated in the form of seromyotomy or conversion to a Roux-en-Y gastric bypass [65].
3.8.2 Porto-Mesenteric Venous Thrombosis
Porto-mesenteric venous thrombosis (PMVT) is an infrequent complication of laparoscopic sleeve gastrectomy . A previous history of VTE is an important predictor for PMVT. It is hypothesized that the division of the short gastric vessels during sleeve gastrectomy with change in blood flow, possible intimal damage of the splenic veins due to direct physical injury while operating in the lesser sac, and dehydration after discharge from the hospital may all contribute to formation of PMVT. The most common symptom on presentation is nonspecific abdominal pain. It may be associated with nausea, vomiting, diarrhea, and gastrointestinal bleeding. Physical examination may vary from low-grade fever, mild abdominal tenderness, and peritoneal signs to florid shock secondary to bowel ischemia. Contrast-enhanced CT scan of the abdomen is diagnostic for this condition. If the patients do not elicit signs of ischemic bowel, therapeutic anticoagulation with heparin is the recommended treatment, which may be subsequently transitioned to oral warfarin. Presence of bowel ischemia without necrosis or perforation may be treated with percutaneous thrombolytic therapy. Bowel necrosis and/or perforation will warrant exploration of the peritoneal cavity with resection of the affected bowel [66]. This raises the debate regarding the use of an antiplatelet agent or an anticoagulant in this patient population to prevent the development of this complication. Due to the relatively rare occurrence of PMVT, the increased risks of bleeding associated with prophylactic therapy should be carefully considered. The authors do not recommend this in their practice as good-quality studies advocating the same in the surgical literature are lacking.
3.9 Acute Complications Specific to Roux-en-Y Gastric Bypass
Gastric bypass has historically been considered as the gold standard procedure for the surgical management of morbid obesity. Until recently, the operation accounted for about 70 % of all bariatric surgeries performed worldwide. The most common early major complications associated with this procedure include anastomotic/ staple-line leaks, postoperative hemorrhage, small bowel obstruction (SBO) due to variable etiology, and marginal ulceration.
3.9.1 Small Bowel Obstruction
SBO following a bariatric procedure is associated with considerable morbidity and mortality if not recognized and treated promptly. Obstruction can be classified into two groups based on the time of presentation after the primary surgery. Early SBO presents within the first 30 days of surgery while late SBO manifests beyond 30 days after surgery. The most common etiology of early SBO is an acute obstruction at the enteroenterostomy, attributed to technical problems with the Roux limb, while internal hernia and adhesive disease are responsible for majority of late SBO. Other causes of obstruction include incisional or port-site hernia , intussusception, anastomotic edema, angulation/kinking of the Roux limb, and hemobezoar. Laparoscopic bariatric procedures have interestingly higher incidence of SBO compared with open approach.
The symptoms related to early-onset SBO might be variable. Obstruction of the Roux limb presents with nausea, heartburn, vomiting, midepigastric pain, and upper abdominal fullness, which may be transiently relieved by emesis. A biliopancreatic limb obstruction, on the other hand, may be associated with nausea, abdominal fullness, tachycardia, hiccups, and shoulder and back pain. Gastric remnant dilation is a pathognomonic sign of biliopancreatic limb obstruction, which can subsequently progress to potential gastric necrosis and/or perforation. Common channel obstruction presents with features of both. Abnormal liver function tests and hyperamylasemia can result from obstruction of both the biliopancreatic limb and the common channel. The history and the physical examination of bariatric patients with early obstruction may often be vague. Hence, a high degree of suspicion and prompt and judicious surgical exploration is advocated to identify and treat these obstructions to prevent disruption of the new anastomosis or staple-line or intestinal necrosis, with subsequent perforation and peritonitis. CT scan with oral contrast is essential for the quick diagnosis of early SBO with the pertinent findings being dilated biliopancreatic limb or gastric remnant, location of small bowel loops in the left upper quadrant, and bowel wall thickening with proximal dilation.
3.9.1.1 Obstruction at Entero-Enterostomy
Technical errors contribute to kinking or obstruction at the enteroenterostomy. Postoperative edema, intraluminal hemorrhage with impaction of large clot at the anastomosis, and angulation of the Roux limb may all precipitate this complication. It leads to a closed-loop obstruction involving the biliopancreatic limb and the gastric remnant, which can be rapidly fatal if not recognized and decompressed [67]. Appropriate orientation of the Roux limb and placement of an anti-obstruction stitch (Brolin stitch) to prevent its kinking may help avoid this complication [68]. Stapled closure of the common enterotomy of these anastomoses can lead to increased incidence of obstruction at this site than hand-sewn closure. Intussusception of the jejunojejunostomy into the Roux limb has also been reported in 0.1–0.3 % patients. Surgical intervention should entail bowel resection and revision of anastomosis as it prevents recurrence [69].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







