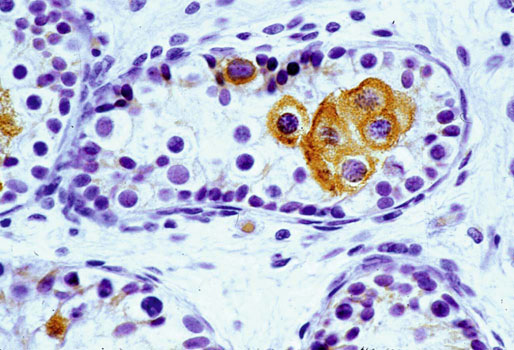
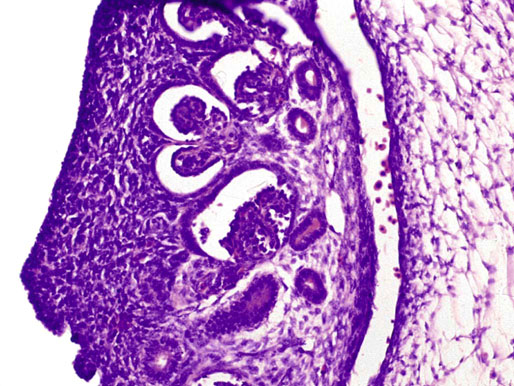
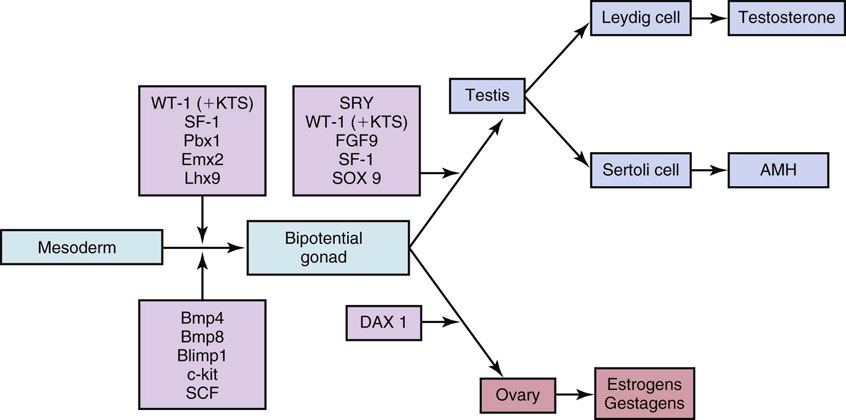
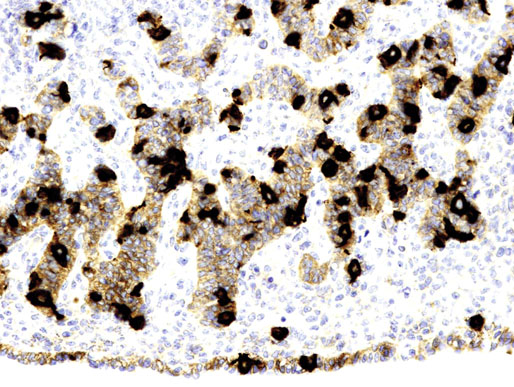
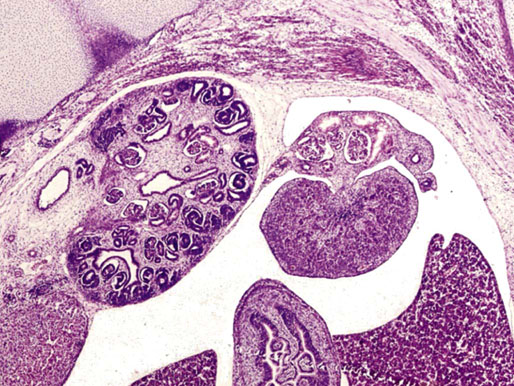
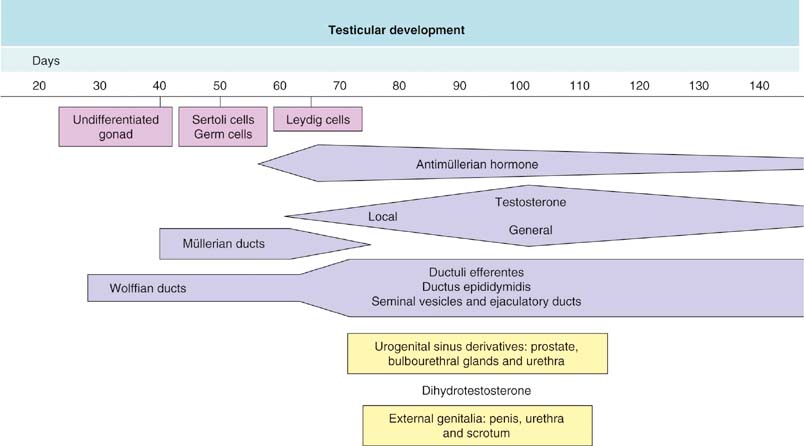
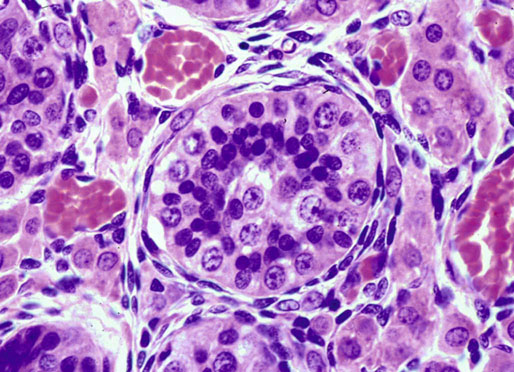
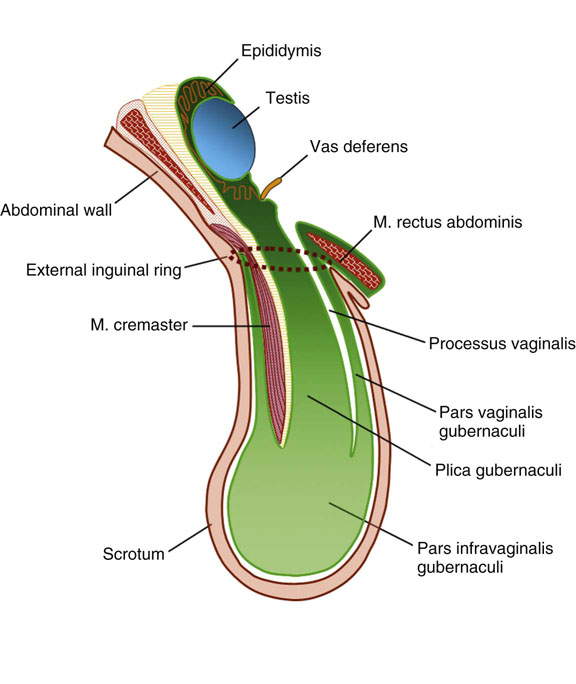
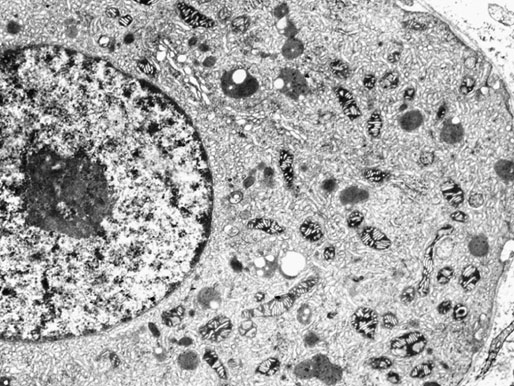
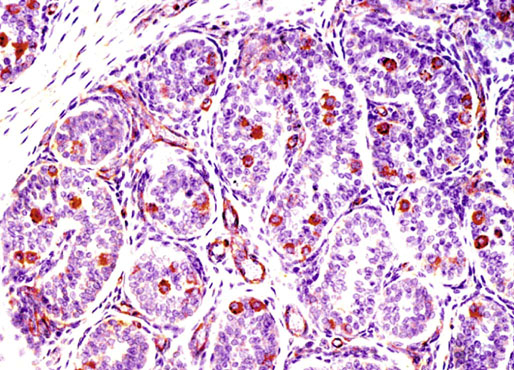
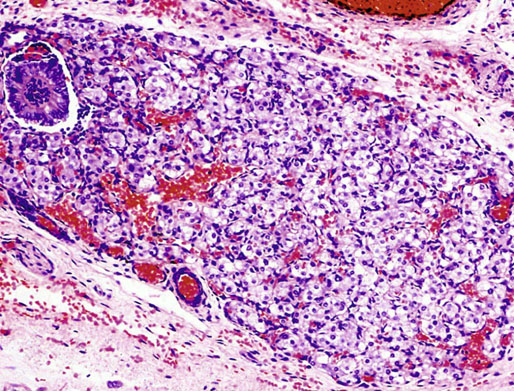
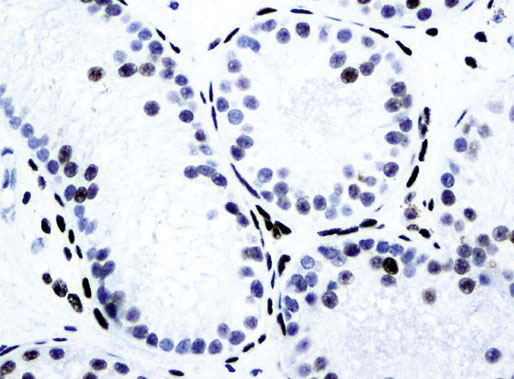
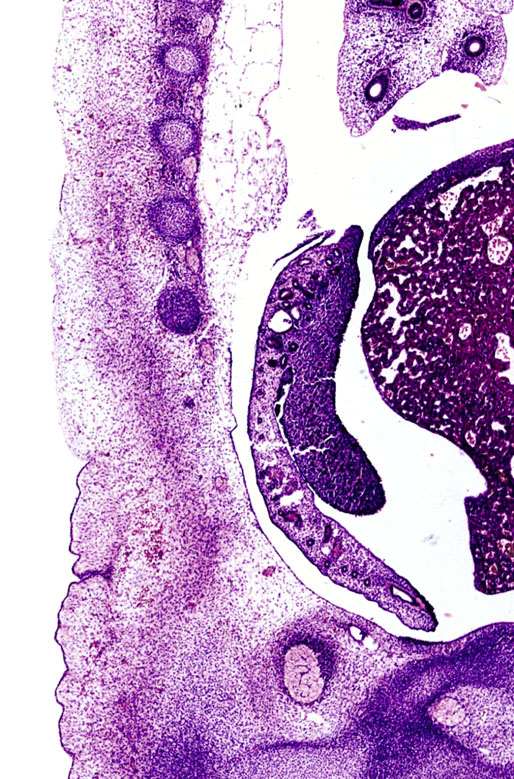
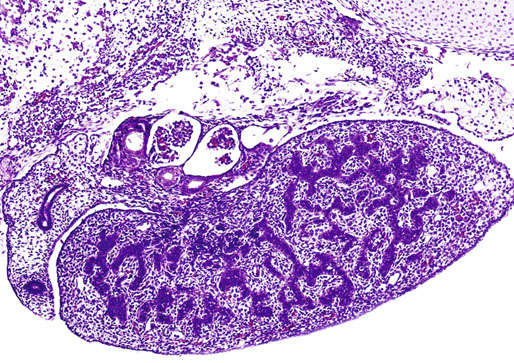
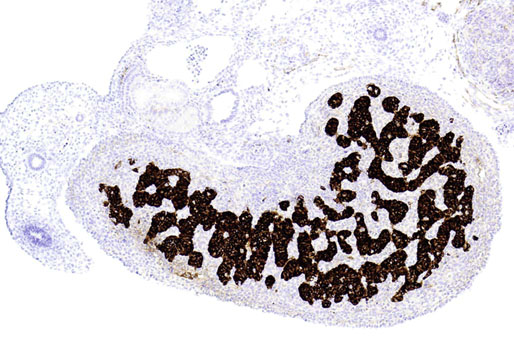
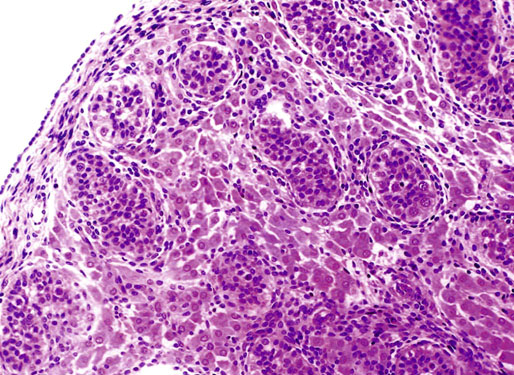
Chapter 12 Embryology and anatomy of the testis Congenital anomalies of the testis Alterations in number, size, and location Hamartomatous testicular lesions Ovotesticular disorder (true hermaphroditism) Infertility and chromosomal anomalies Other syndromes associated with hypergonadotropic hypogonadism Secondary idiopathic hypogonadism Hypogonadism secondary to endocrine gland dysfunction and other disorders Infertility secondary to physical and chemical agents Other testicular and epididymal lesions Epididymitis induced by amiodarone Ischemic granulomatous epididymitis Scrotal calculi, scrotal pearl Sexual differentiation is the result of complex genetic and endocrine mechanisms that are closely associated with the development of both the genitourinary system and the adrenal glands. Formation of the bipotential gonad—and subsequently, of the testis or the ovary—depends on gene expression in both sex and autosomal chromosomes. Testes secrete steroid and peptide hormones, which are necessary for the development of inner and outer genitalia. These hormonal actions are mediated by specific receptors that function as transcription regulators. Alterations of these genetic events lead to sexual dimorphisms involving the inner and outer genitalia and can also hinder the development of other organs.1 In addition, the coelomic epithelium proliferates to invade the subjacent mesenchymal tissue. Cell proliferation at this phase also depends on Lhx9 expression. The basement membrane underlying the coelomic epithelium appears discontinuous and is rich in laminin content. As the coelomic cells proliferate, laminin production in the gonadal ridge increases; this laminin seems to be essential for germ cell colonization. Immediately beneath the coelomic epithelium are several mesonephric tubules and glomeruli (Figs. 12-1 and 12-2). Fig. 12-1 Longitudinal section of a fetus showing the primitive gonad as an elongated structure along mesonephros. In the upper right corner of the image the lung can be recognized and the liver is in front of the gonad. The primordial germ cells do not arise within the genital ridge or the mesonephros, but rather migrate from another location. Initially, the genital ridges are devoid of germ cells. Primordial germ cells can already be detected in the third week of gestation in the extraembryonal mesoderm that lines the yolk sac posterior wall, near the allantoic evagination. The appearance of these cells is associated with the expression of several proteins, such as Bmp4/Bmp8 and Blimp1, in the extraembryonal mesoderm. Primordial germ cells are ovoid, measuring 12 to 14 µm in diameter, and are easily detected immunohistochemically by a high content of alkaline phosphatase. The nuclei are spherical and possess one or two large and prominent central nucleoli.2 The cytoplasm contains mitochondria with tubular cristae, lysosomes, microfilaments, lipid inclusions, numerous ribosomes, and abundant glycogen granules. Attracted by chemotactic factors, the primordial germ cells migrate along the mesenchyma of the mesentery and reach the genital ridge by 32 to 35 days.3,4 Primordial germ cells start to express the two membrane proteins fragilis and brachyurus, and the cells migrate through the primitive streak to settle into the developing endoderm (hindgut).5 The hindgut then invaginates into the future abdominal cavity and approaches the gonadal ridges. Primordial germ cells migrate by ameboid movements along the hindgut mesentery to reach the gonadal ridges. This migration process requires the interaction of several factors: the integrin CXCR4-β1 (expressed by primordial germ cells), stromal cell–derived factor 1 (SDF1, expressed by the body wall mesenchyma and gonadal ridges), and several extracellular matrix proteins.6–9 An essential mechanism for adequate primordial germ cell migration, survival, and chemoattraction is the interaction between the protein c-kit (CD117), expressed in germ cell surface, and stem cell factor (SCF) present in the surrounding tissues.10–12 After entering the gonadal ridges, primordial germ cells colonize them; this process involves the expression of E-cadherin and germ cell interaction with a rich laminin network produced by organizing coelomic cells in the gonadal ridges.13 The association of coelomic-derived somatic cells, primordial germ cells, and a laminin-rich stroma in the gonadal ridge characterizes the gonadal blastema. Once inside the genital ridge, germ cells lose their motility and begin to aggregate. Normal male determination depends on the expression of the SRY (sex-determining region Y) gene, located on Y chromosome. In the absence of SRY, an ovary is formed. In the testicular blastema, SRY is exclusively expressed by those coelomic-derived somatic cells14–16 induced to differentiate into pre–Sertoli cells, which form the sex cords. These cells are believed to act as the organizing center of the male gonad and to orchestrate the differentiation of all other cell types. SRY expression is transient, ceases when sex cords start to be formed, and is activated by WT1 (+KTS isoform), which is consistently expressed in the coelomic epithelium and the proliferating coelomic-derived somatic cells.17,18 Gonads lacking WT1 (+KTS) show lower SRY levels per cell and also fewer SRY-positive cells. This observation led investigators to hypothesize that WT1 (+KTS) contributes to SRY activation not only in a cell-autonomous manner, but also in a non–cell-autonomous manner, by increasing the number of pre–Sertoli cells.19 SRY expression is observed first in the anterior and central portions of the gonad and then in the poles.15 Expression of SRY requires a proliferating population of the gonadal somatic cells, and both SF1 and fibroblast growth factor 9 (FGF9) play a role in this proliferation.20,21 Immediately after SRY expression has started in the gonad, FGF9 contributes to maintaining cell proliferation, necessary for sex cord formation.22,23 In particular, FGF9 regulates the male-specific proliferation that produces pre–Sertoli cells.24 The beginning of sex cord formation, and thus the first morphologic distinction between a testis and an ovary, also depends on the expression of SRY-box containing gene 9 (SOX9). This expression occurs in the cytoplasm of somatic elements in the bipotential gonadal ridge. SOX9 is expressed in the pre–Sertoli cells in the same dynamic wave as SRY: it originates in the center of the gonad and then continues to the rostral and caudal poles. Its transcription is activated by the synergistic action of SRY and SF1. SOX9 also stimulates other factors that lead to the differentiation of Sertoli cells such as FGF9 and prostaglandin D2 (PGD2).25 Although SOX9 is also expressed in the female gonad, there are important differences with regard to the male gonad.16,17,26,27 In males, unlike in females, SOX9 gene transcription increases, and its protein product translocates into the nucleus. This event occurs simultaneously with the initiation of sex cord formation. Therefore, like SRY, SOX9 is necessary and sufficient for both Sertoli cell differentiation and testis development.28 SOX9 expression in pre–Sertoli cells remains after sex cord formation, a finding indicating that SOX9 may have additional roles during proliferation and maturation of the testis, although it is dispensable for the development of embryonic and early postnatal testis.29 Contacts among pre–Sertoli cells during sex cord formation are regulated by neurotropic tyrosine receptor kinases (NTRKs) (Fig. 12-3).30 Somatic coelomic epithelium-derived cells showing nuclear SOX9 expression start to organize into tubular structures that form the primordial sex cords.14,17,26 These pre–Sertoli cells acquire epithelial characteristics, polarize their organelles, synthesize basement membrane,31–33 and express antimüllerian hormone (AMH) under the synergistic action of SOX9 and SF1.17,34 During this period, migrating primordial cells start to aggregate with pre–Sertoli cells in the primordial cords. The initial development of the testis requires specific integrations among germ cells, pre–Sertoli cells, endothelial cells, and interstitial cells. Shortly after formation of the gonadal blastema in male gonads, some cells of the adjacent mesonephros start to migrate to the gonadal blastema. This migration depends on the SRY expression by coelomic-derived cells and is controlled by the expression of FGF9. The mesonephros contributes several cellular types to gonad development. Endothelial cells originate from the gonad-mesonephros border and differentiate into vessels as they migrate more deeply into the gonadal blastema. Parallel to the organization of seminiferous cords, vascularization of the testis is developed and mediated by platelet-derived growth factor receptor α (PDGFRα) and vascular endothelial growth factor (VEGF) through adequate formation and branching of the coelomic vessels.35,36 The action of VEGF and endothelial cells is a requirement for Sertoli cells to organize in testis cords.37,38 Perivascular cells migrate into the gonad from the mesonephric border along with endothelial cells, and these vessel-associated cells likely represent an interstitial precursor that will give rise both to Leydig cell progenitors and to the remaining types of interstitial cells.39 These Leydig cell progenitors express LHX9 (LIM homeobox gene 9) but no gonad somatic markers such as transcription factor GATA-4 and SF1. The vessels not only contribute to Leydig cell progenitor migration but also affect their proliferation.40 Leydig cells originate from mesonephric cells and also directly from coelomic epithelium.22,33 These progenitor cells express LHX9 as well as gonadal somatic markers. Fetal Leydig cell differentiation has been shown to be regulated, at least in part, by three signaling molecules or pathways: desert hedgehog (DHH), platelet-derived growth factor A (PDGFA), and Notch signaling.41 Myoid peritubular cells comprise another cell type that appears early. Although these cells are not involved in the initial portioning of the XY gonad into cord regions of clusters of Sertoli and germ cells, myoid peritubular cells interact with pre–Sertoli cells to promote basal lamina deposition and tubular organization.32,42 Once primordial germ cells (that have proliferated in the seminiferous cords) undergo mitotic arrest in the G1 and G0 stages of the cell cycle, they are called gonocytes. These cells remain in mitotic arrest until a few days after birth, when they resume proliferation. This mitotic arrest depends on adequate cord formation and is probably mediated by inhibitory signals provided through gonocyte interactions with Sertoli cells.43 Initially, gonocytes are located in the central (“luminal”) portion of seminiferous cords. Later, during the fetal and neonatal periods, gonocytes initiate active intracordal migration toward the cord basement membrane. This process is secondary to gonocyte–Sertoli cell adhesion mediated by neural cell adhesion molecule (NCAM).44,45 Gonocytes resume mitosis as soon as migration starts; mitoses are patent in the gonocytes that have reached the basement membrane. These divisions result in the first generation of spermatogonia. Gonocytes that fail to migrate to the basement membrane undergo apoptosis. It has been suggested that AMH plays a role in gonocyte migration and the start of mitotic activity.46 Pre–Sertoli cells differentiate at approximately the end of the seventh week from somatic cells in the sex cords, which should now be designated seminiferous cords. It was formerly assumed that that interaction of peritubular myoid cells and pre–Sertoli cells was essential for seminiferous cord formation, to promote basal lamina deposition and tubular organization.32,42 However, more recent evidence supports the premise that peritubular myoid cells are not involved in the initial partitioning of the XY gonad into cord regions, which consist of clusters of both pre–Sertoli cells and germ cells. As soon as seminiferous cords are formed by the interaction of peritubular myoid cells and pre–Sertoli cells, primordial germ cells become “entrapped” in those tubules. This entrapment is mediated by interactions between primordial germ cells and pre–Sertoli cells through expression of E-cadherin and P-cadherin in the surface of both cell types.13 The differentiation of pre–Sertoli cells into Sertoli cells is manifested by the polarization of these cells, when they form aggregates that assemble into seminiferous cords. Early events include the following: the development of intercellular junctions between adjacent Sertoli cells; the formation of a basal lamina that surrounds the external surface of seminiferous cords; and the expression of AMH, SPG-2 (sulfated glycoprotein-2), and clusterin by the Sertoli cells.47 It has been shown that activin A, the major transforming growth factor-β (TGF-β) protein, produced by fetal Leydig cells, acts directly on Sertoli cells to promote their proliferation during late embryogenesis and plays an essential role in seminiferous cord morphogenesis in murine testis48 (Figs. 12-4 to 12-6). Fig. 12-4 Longitudinal section of an 8-week-old fetal testis showing sex cord configuration. In the hilum are several glomeruli and nephric tubules. Fig. 12-5 An 8-week-old fetal testis showing intense expression of inhibin in pre–Sertoli cells that form sex cords. Because peritubular myoid cells express many genes in common with interstitial cells from early fetal development, it has been hypothesized that peritubular cells have an interstitial origin either from the mesenchymal cells that populate the initial genital ridge or from the somatic cells that proliferate from the coelomic epithelium.37 Peritubular myoid cells form a single layer of flattened cells that surround the Sertoli cells and circumscribe the seminiferous cords. Basal lamina formation by peritubular myoid cells is regulated through DHH (DHH homologue gene) expression by the myoid cells themselves.49 Survival of peritubular myoid cells, and therefore seminiferous cord formation, depends on DAX1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1) nuclear receptor expression, induced in turn by SF1 expression.50–52 DAX1 expression ceases in seminiferous cords after they are formed, whereas it is maintained in ovaries, a finding suggesting that dosage and stage-specific expression of this protein may be responsible for ovarian differentiation. Seminiferous cords lose their connection with the coelomic epithelium, whose height decreases to one or two cell layers. Leydig stem cells proliferate actively and start their differentiation in the eighth week of gestation. The detection of 3β-hydroxysteroid dehydrogenase (3β-HSD) by histochemical methods is considered the first signal of differentiation, which becomes complete when the cells acquire the ultrastructural characteristics of steroidogenic cells. This differentiation is independent of hormonal secretion and is induced by two Sertoli cell–derived signaling molecules: DHH and PDGFA.53 Other factors involved in the control of the development and functions of fetal human Leydig cells are GATA-4 (transcription factor that recognizes the GATA consensus DNA sequence), insulin-like growth factor-1 (IGF-1),54 and the basic helix-loop-helix transcription factor POD1; GATA-4 and IGF-1 are stimulating factors, whereas the POD1 is involved in suppression of fetal Leydig cell differentiation.53 As fetal development progresses, new cells differentiate from precursor cells located in the peritubular cell layer, specifically in the outer of the two layers present between the seventeenth and twenty-second weeks of fetal life (weeks 19 to 24 of gestational age). After the twenty-second week of life the tubular wall is reduced to the internal layer. The differentiating Leydig cells can be identified because they are the only cellular type that expresses the androgen receptor (AR) at this stage of development. During the sex differentiation period, testosterone production and its conversion to a more active metabolite (dehydroandrosterone) comprise a gonadotropin-independent process.55 Not until the second half of gestation, when the hypophyseal receptors to luteinizing hormone (LH) develop, do gonadotropins gain importance. Rete testis develops from residual cell cords that persist from the mesonephros in continuity with seminiferous cords. The mesonephros and its testicular connection become progressively thinner and show a circular outline in cross sections. The testis remains between two ligaments: the cranial suspensory ligament and the caudal ligament. The caudal ligament gives rise to the gubernaculum (Fig. 12-7). The development of the urogenital tract also begins at the stage of the undifferentiated gonad, with the appearance of two different pairs of ducts: the wolffian ducts and the müllerian ducts (Fig. 12-8). Wolffian ducts arise inside the mesonephros; this explains the close relationship between the reproductive and urinary systems. This pair of ducts originates in the third week of gestation, when the cranial region of the segmented intermediate mesoderm gives rise to 10 pairs of tubules, the nephric tubules, arranged with a segmental distribution. One end of each nephric tubule opens to the coelomic cavity, and the other end empties into an excretory duct. There are thus two excretory ducts, longitudinally placed at both sides of the embryonal axis. This excretory system is named pronephros and has a short life. In the fourth week the pronephros disappears and is replaced by another tubular excretory system, the mesonephros, derived from nonsegmented intermediate mesoderm. The medial ends of the mesonephric tubules do not open to the coelomic cavity but are connected to glomeruli at one end; at the other end, the excretory ducts are the two wolffian ducts. The caudal ends of the wolffian ducts drain into the urogenital sinus.56 At the end of the second month of gestation, the mesonephros is replaced by the metanephros, or definitive kidney. However, in the male, the most caudally located mesonephric tubules remain to give rise to the efferent ducts, whereas the wolffian ducts are the source of the epididymides, vas deferens, seminal vesicles, and ejaculatory ducts. Both müllerian ducts originate from two longitudinal invaginations of the coelomic epithelium in the anterolateral aspects of both genital ridges. The cranial end of each duct is a funnel that opens into the coelomic cavity. The initial segments of both ducts run parallel and lateral to their respective wolffian ducts, and as they pass caudally, the müllerian ducts cross over the wolffian ducts to lie medial to them. Finally, in their distal portions, both müllerian ducts fuse into a single duct that will give rise to the uterovaginalis duct. This duct elongates caudally up to reach the posterior aspect of the urogenital sinus, where the duct forms a dilation named the Müller tubercle. Each wolffian duct drains at one side of this tubercle.57 The later developmental stages of the male genital system are under hormonal control. In general, the mammalian fetal testis undergoes an early period of independence regarding hormonal control and then becomes LH (and possibly follicle stimulating hormone [FSH]) dependent in the second half of gestation.58 The most important hormones in this context are AMH, testosterone, dihydrotestosterone (DHT), and the pituitary hormones FSH and LH. AMH, also called müllerian-inhibiting substance (MIS),59 is secreted by Sertoli cells and is a glycoprotein polymer consisting of two identical subunits (each of 72 kDa), linked by a disulfide bridge.60–62 AMH is a member of the TGF-β superfamily and is synthesized as a precursor peptide (560 amino acids) with proteolytic cleavage at 109 amino acids from the C terminus, which is required for hormone activation. AMH is encoded by a 2.75-kb gene, which comprises 5 exons and is located in the p13.3 region of chromosome 19.63–65 AMH is secreted only by somatic gonadal cells that include male Sertoli cells and female granulosa cells. This hormone is detected from the sixth week of development (eighth to ninth week of gestation), probably as soon as primordial germ cells contact the Sertoli cell precursors. This event occurs exactly 1 week before the müllerian ducts lose their responsiveness.66,67 AMH is at high concentration during the second trimester and drops markedly in the third trimester.68 These levels increase again noticeably during the first year of postnatal life and decrease again during infancy and childhood. At the onset of puberty, AMH drops dramatically to very low or undetectable levels, which persist through adult life. The amount of hormone secreted by Sertoli cells is inversely correlated with the maturation degree of these cells.59,69,70 The regulation of AMH production is only partially understood. Its expression is regulated by SOX9; SF1 also seems to be involved.71 SF1 (also called Ad4BP) is an orphan nuclear receptor, which acts as a transcriptional regulator of all the steroidogenic genes that form the P450 complex. It also has a regulating effect on the SRY factor because SRY expression in Sertoli cells is detected shortly before AMH expression can be detected.72 During puberty, AMH is negatively regulated by androgen levels.73 AMH acts on the testis and genital tract, as well as on extragenital structures. It causes involution of the ipsilateral müllerian ducts. In each duct, the action is initiated in the end nearest to the caudal extreme of the testis and progresses rapidly along the duct. In adulthood, remnants of this duct can be observed near the cranial (testicular hydatid) and caudal (prostatic utricle or verumontanum) ends of the testis. AMH is also responsible for tunica albuginea formation. This occurs by accumulation of mesenchyma between the coelomic epithelium and the sex cords. This mesenchyma gives rise to well-collagenized connective tissue, which contains several layers of fibers that are arranged parallel to the testicular surface.74 AMH also hinders spermatogonial proliferation into meiotic spermatocytes,75 and it has a paracrine role regulating fetal androgen production.76 The most important extragenital function of AMH is maturation of fetal lungs.77 Testosterone synthesis by Leydig cells is regulated by two hormones: human chorionic gonadotropin (hCG) and LH; one thirtieth of hCG production enters the fetus. This secretion reaches a peak from the eleventh to the fourteenth week, preceding the highest secretion of testosterone, which occurs from the eleventh to the seventeenth week. From the eighteenth week onward, hCG drops markedly. The hCG-dependent testosterone production plays an important role in genital differentiation. Wolffian duct differentiation occurs only in response to testosterone secretion by the ipsilateral testis, and this differentiation gives rise to the ipsilateral epididymis, vas deferens, and seminal vesicle.78,79 Anomalies of androgen synthesis during embryogenesis lead to incomplete masculinization and cryptorchidism. DHT is formed from testosterone by the action of the enzyme 5α-reductase and causes the differentiation of the prostate and the development of the external genitalia: male urethra, penis, and scrotum. The scrotum is formed by the fusion of both labioscrotal folds in their midline. The fusion line is named the scrotal raphe. The penile urethra, which up to this time has been a urethral groove, is formed by the fusion of urethral folds. The genital tubercle enlarges to form the glans penis. The terminal segment of the penile urethra is derived from an ectodermic invagination of the glans end. The urogenital sinus gives rise to the urinary bladder, the prostatic urethra, and the prostate.57 The first effects of DHT, which include fusion of labioscrotal folds and medial raphe closure, are observed from day 70; on approximately day 74 the urethral groove is closed; and between the eighteenth and twentieth weeks the development of external genitalia is complete.80 The fetal hypophyseal hormones FSH and LH play important roles in the last months of gestation. LH is not found in fetal circulation up to the tenth week, and its peak is reached in the eighteenth week. Thereafter, this secretion decreases slowly until birth. LH controls androgen production in the second half of fetal life; fetal Leydig cells are devoid of LH receptors (LHRs) in the first half of gestation. Conversely to what happens in adults, LH does not exert negative control over LHRs and androgen production by fetal Leydig cells.55 The steroidogenic ability of fetal Leydig cells is higher than that of adult Leydig cells. Fetal Leydig cells are insensitive to estrogen inhibitor effects. FSH acts as an essential mitogen factor for Sertoli cells, which undergo maximal mitotic activity at the end of fetal life.81,82 This hormone seems to activate transcription factors such as GATA-4, which shows intense Sertoli cell expression from the nineteenth to the twenty-second week, that is, shortly after a rise in fetal serum FSH. GATA transcription factors are structurally related zinc finger proteins that recognize a consensus DNA sequence (A/T)GATA(A/G), known as GATA motif, which is an essential cis-acting element in both promoters and enhancers of a variety of genes.83 The structure of fetal testis evolves under the influence of placental hormones and the fetal hypophysis. Testicular changes involve modifications in outer morphology (testicular shape changes from elongated to ovoid), as well as the development and differentiation of the different testicular cell types. The degree of development is parallel in both testes, and testicular growth depends on gestational age.84 The testis becomes covered by the tunica albuginea early in gestation. The tunica albuginea increases in thickness by 10-fold from the tenth to the forty-first week of gestation. From the twenty-ninth week onward, two layers can be distinguished in the tunica albuginea: an outer fibrous layer and an inner loose layer. Interlobular septa begin to appear between the seventeenth and twenty-first weeks and are completely formed between the twenty-fifth and twenty-eighth weeks. These septa support blood vessels. Nerve fibers are seen for the first time in the sixteenth week within the loose connective tissue of the albuginea (tunica vasculosa) and in the twentieth week in the testicular septa.85 These irregularly outlined, compact structures gradually acquire a cylindrical shape and become longer and convoluted. Their diameter increases very slowly up to the sixteenth week and is maintained up to birth. During the fetal life, the seminiferous cords consist of Sertoli cells and germ cells and are surrounded by a tunica propria. The seminiferous cords are solid structures devoid of lumina. Among the cords, the connective tissue forms the testicular interstitium, which contains numerous Leydig cells86 (Fig. 12-9). Conversely to what occurs in other species, in human fetal testis, germ cells are not a homogeneous population. Ultrastructural studies have identified several cell types that form the basis of different classifications.87–90 Three germ cell types have been distinguished in the fetal testis by immunohistochemical studies: gonocytes, intermediate cells, and fetal spermatogonia (prespermatogonia). Gonocytes are identified as isolated small cells located in the center of the seminiferous cords during most of fetal life. Their nucleus is spherical and possesses a voluminous, centrally located nucleolus.91,92 Their cytoplasm shows a well-developed Golgi complex, lipid droplets, short rough endoplasmic reticulum cisternae, and microfilaments. These microfilaments are present mainly beneath the cell surface. The gonocyte immunohistochemical profile is positive for octamer-binding transcription factor 4 (OCT4), c-kit, placental alkaline phosphatase (PLAP), and checkpoint kinase 2 (CHK2) and negative for melanoma-associated antigen 4 (MAGE-A4). Gonocytes are also proliferating cell nuclear antigen (PCNA) positive, a finding that suggests active cell proliferation.93–95 The germ cell number per cross-sectioned cord reaches a peak between the twelfth and twenty-second weeks.96 At the tenth week, most of germ cells are gonocytes; at approximately the fifteenth week, many intermediate cells are present together with gonocytes, and fetal spermatogonia can be observed for the first time. From the sixteenth to the twentieth week, an important germ cell degeneration occurs,97 and the degenerated cells are phagocytosed by Sertoli cells. From the twenty-second week onward, most germ cells are fetal spermatogonia. Mitotic activity is high in the last trimester of gestation.98 Approximately 22% of fetal testes at the age of 14 to 33 weeks show ectopic germ cells located beneath the coelomic epithelium, in the connective tissue that separates the testis from the epididymis, or in the rete testis.99 Fetal Sertoli cells are the most numerous cells in the seminiferous cords, where they form pseudostratified epithelium that rests on the basal lamina. At approximately the thirteenth week of gestation, the Sertoli cells exhibit an indented outline and long cytoplasmic processes and are interconnected by desmosomes. The nucleus is spherical and contains a small nucleolus. The cytoplasm is electron dense and contains numerous lysosomes, actin microfilaments, and intermediate filaments. Other organelles observed are microtubules, mitochondria, and a well-developed Golgi complex. In the apical region are numerous, parallel rough endoplasmic reticulum cisternae. The Sertoli cells progressively elongate, their cytoplasm becomes less electron dense, and filaments predominate in the basal region.3 Intermediate filaments of the vimentin type are expressed throughout their whole life, whereas low-molecular-weight cytokeratins (numbers 8, 18, and 19) are present only up to the twentieth week.100,101 Desmin filaments have been observed only from the eleventh to the fourteenth week.102 Fetal Sertoli cells express inhibin.103 During gestation, the number of Sertoli cells increases even though mitosis is only occasionally observed. Although Sertoli cell numbers per cross-sectioned cord do not increase during this period, Sertoli cell proliferation contributes to the increased length and tortuosity of seminiferous cords. Both Sertoli cell proliferation and testicular cord expansion take place by the action of activin A, which is secreted by Sertoli cells. At the end of gestation, there are approximately 260 million Sertoli cells per pair of testes. In the fetal and early postnatal period, the absence of AR expression in Sertoli cells underlies a physiologic stage of androgen insensitivity within the male gonad in this period.104 Among the multiple fetal Sertoli cell functions, the following are worth highlighting: AMH secretion, fetal Leydig cell differentiation induction,105 and prevention of the entry of germ cells into meiosis.106 From the fourteenth week, two types of peritubular cells can be observed: inner myoid cells and fibroblast-like cells. Fibroblast-like cells occupy the outermost layers. In total, there are four to five layers of peritubular cells. At this time the number of myoid cells is very low, but at the thirty-fourth week these cells predominate. The probable precursors of myoid cells are the fibroblast-like cells because both cell types express the Ki67 antigen.107 In the last weeks of gestation, the number of peritubular cell layers decreases to only two. This could be attributed to the intense lengthening of seminiferous cords at this time and the Leydig cell differentiation from their peritubular cell precursors. The presence of the AR in peritubular myoid cells suggests an important role in Sertoli cells.108 These cells appear among the seminiferous cords from the eighth week of gestation, and their number increases up to 48 million per pair of testes (≈50% of testicular volume at this moment) between the thirteenth and sixteenth weeks,55 coinciding with a testosterone peak109 (Fig. 12-10). This Leydig cell number is maintained up to the twenty-fourth week, although the testicular volume occupied by Leydig cells is lower at this time because the seminiferous cords have grown markedly during this period. From the twenty-fourth week up to birth, the Leydig cell number progressively decreases to 18 million per pair of testes.58,97,110 Fig. 12-10 A 16-week-old fetal testis showing numerous Leydig cells in the testicular interstitium and slightly convoluted seminiferous tubules. Leydig cells are polyhedral and measure between 30 and 37 µm in diameter. They have an eccentric and pale nucleus, with a voluminous nucleolus, and an eosinophilic cytoplasm. Their ultrastructural pattern is characterized by the abundance of smooth endoplasmic reticulum, numerous mitochondria with tubular cristae, and a variable number of lysosomes and lipid droplets. The rough endoplasmic reticulum consists of some groups of a few short, parallel cisternae.111 These cells differ from adult Leydig cells by the absence of both Reinke crystals and paracrystalline structures and by the lesser amount of lipid droplets.112 Fetal Leydig cells show positive histochemical reactions to acid phosphatase, glucose-6-phosphatase, and 3β-HSD. In addition to testosterone, these cells secrete several peptides that play important roles in the endocrine and paracrine mechanisms in the control of testicular function.113 One of these peptides, insulin-like factor 3 (INSL3), is important in testicular descent.114 Most fetal testes (71.9%) receive blood through three arteries: the testicular (inner spermatic) artery, which originates from the abdominal aorta; the deferential (vassal) artery, which originates from the inferior vesical artery; and the cremasteric (outer spermatic) artery, which is a branch of the inferior spermatic artery. In 23.4% of fetal testes, only two arteries (testicular and deferential) are present, and 4.7% of testes have four arteries.115 Although the testis and epididymis form an anatomic and functional entity, the anatomic relationships between testis and epididymis vary widely. The most frequent presentation (89.72%) is an epididymis connected to the testis by the caput and cauda of the epididymis, with the corpus epididymidis remaining separate from the testis. In other cases (7.53%) the epididymis is closely attached to the testis by all its parts (caput, corpus, and cauda). Finally, a few cases (2.75%) have deficiencies in the testis-epididymis junction, either in the caput or the cauda.116 These epididymal presentations are related neither to the position of the testis nor to the side of the body (right or left). During fetal life, androgenic receptors are observed in epithelial cells of both the efferent ducts and the epididymal duct, as well as in the peritubular stroma.108 Testicular descent is the result of hormonal and mechanical actions that are not fully understood.117 The process of testicular descent begins after the eighth week of gestation and not later than the fifteenth week; the descent accelerates from the twenty-fourth to the twenty-sixth week.118 At the twenty-third week, most testes (90%) are still in the abdomen and, from the twenty-sixth to the twenty-eighth week the testes pass through the deep inguinal ring. Testicular displacement through the inguinal canal lasts a few days. At approximately the twenty-eighth week, the testes pass through the superficial inguinal ring and reach the scrotum; this process is completed in 3 to 4 weeks. After the thirty-fifth week, the testes should have resumed their descent to the scrotum in normal fetuses.119 Three phases are classically recognized in testicular descent: nephritic, transabdominal, and inguinal. In nephric displacement, the gonad detaches from the metanephros (primitive kidney); this process is completed in the seventh week. Transabdominal descent consists of the displacement of the testis from the posterior abdominal wall to the neighborhood of the future inguinal region (inner inguinal ring, also called the deep ring); this occurs between the eighth and fifteenth weeks. This displacement is associated with regression of the cranial suspensory ligament and enlargement of the caudal suspensory ligament (gubernaculum). At the same time, marked growth of the lumbar backbone takes place, and as a result the testis moves away from kidneys.120 Inguinal descent refers to the entry of the testis into the inguinal canal and its complete descent up to the scrotal pouch, and it occurs between the seventh month of gestation and birth. Gubernacular thickening occurs from the sixteenth to the twenty-fourth week of gestation and is produced by an increase in the number of cells, as well as in the amount of glycosaminoglycans and hyaluronic acid.121 This tissue later absorbs water that determines the final volume of the gubernaculum. The tissue is reminiscent of Wharton jelly of the umbilical cord. By this time, the testis-epididymis complex is pear shaped, and its largest component is the gubernaculum. The inguinal descent of the testis behind the gubernaculum begins in the twenty-fifth week. The testis and epididymis slide through the inguinal canal behind the gubernaculum. Simultaneously, development of the processus vaginalis is completed, and the gubernaculum begins to shorten, becoming fibrous tissue located caudal to the testis and epididymis (gubernacular regression)117; the epididymis develops further, and the testicular blood vessels and vas deferens lengthen (Fig. 12-11). The critical role of normal hormonal function in testicular descent is supported by many clinical and experimental observations.122 In laboratory animal models, destruction of the pituitary blocks testicular descent. Anencephalic fetuses and patients with familial hypogonadotropic hypogonadism usually have undescended testes. Many cryptorchid patients have transient neonatal hypogonadotropic hypogonadism. Some cryptorchid testes descend after treatment with hCG or gonadotropin-releasing hormone (GnRH). Patients with absent LH and ARs have undescended testes. Another important prerequisite for the testis to leave the abdominal cavity and enter the inguinal canal is adequate abdominal pressure.123–125 For example, in the prune-belly syndrome, abdominal bilateral cryptorchidism is associated with urologic malformations and lack of abdominal wall musculature. In a variant of this syndrome termed pseudo–prune-belly syndrome, a positive correlation is seen between the development of abdominal wall musculature and testicular descent. The more developed the abdominal wall musculature is, the lower the situation of the testes will be.126 The development of the processus vaginalis also plays a critical role in testicular descent. Growth of the processus vaginalis into the gubernaculum takes place harmoniously. If this structure is invaded, even partially, by fibrous tissue, the testis will descend in an abnormal direction, thus giving rise to testicular ectopies. If fibrous tissue completely replaces the gubernaculum, the processus vaginalis and cremasteric muscle will fail to develop fully, and as a result the testis will remain mechanically blocked in its route of descent.127 Given that nephric displacement consists only of detachment of the gonad from the mesonephros, testicular descent has been described to occur in two main phases, each regulated by different factors. The most important factor for transabdominal displacement is the androgen-independent peptide INSL3 (also called INSF3 or IGF-3), a member of the relaxin-insulin family that is produced by fetal Leydig cells.128–131 This peptide stimulates the gubernaculum cells to initiate gubernacular swelling.132–136 In experimental animal models, mutations in the genes that encode INSL3 or its receptor LGR8 (leucine-rich repeat-containing G-protein–coupled receptor 8), or another receptor called RXFP2 (relaxin/insulin-like family receptor 2), cause cryptorchidism by disrupting the transabdominal phase of descent.137,138 In humans, however, mutations in the genes that encode INSL3 or its receptors have been found in only 1% of cryptorchid patients, even in studies of familial cryptorchidism.139–141 The low frequency of these mutations in human cryptorchid patients may be linked to the finding that the first phase of testicular descent in humans is seldom disrupted, but the inguinoscrotal phase is usually impaired.140 Analyses of other potential candidate genes for human cryptorchidism, such as the homeobox genes HOXA10 and HOXA11, and the estrogen receptor ESR1, have similarly failed.141 Androgens facilitate regression of the cranial suspensory ligament, which also seems to contribute to positioning of the gonad. In contrast, the inguinoscrotal phase of testicular descent depends on androgenic action, as explained by the genitofemoral nerve hypothesis.142,143 Based on this hypothesis, androgens would act on the genitofemoral nerve nuclei, located in the spinal cord, rather than directly on the gubernaculum. Under androgenic action, genitofemoral nerve neurons would undergo masculinization.142 In male mice, the number of these neurons is higher than in female mice, and the neurons secrete calcitonin gene–related peptide (CGRP), which is the genitofemoral nerve neurotransmitter. The gubernaculum tip may contain an area of primitive mesenchymal cells. The growth of the gubernaculum is the result of two opposite facets: cell proliferation and apoptosis. In experimental studies, CGRP induces mitosis and also prevents apoptosis.144 Another effect is the induction of rapid rhythmic contractions of the gubernaculum. It has been suggested that the range of genitofemoral nerve–mediated androgenic effects is wider and also involves obliteration of the processus vaginalis, inguinal canal differentiation, differentiation of cremaster muscle myocytes,117 and even the beginning of transabdominal descent through involution of the testicular cranial suspensory ligament. Other factors in testicular descent are epidermal growth factor (EGF) and estrogens.145,146 EGF has a positive effect on testicular descent through stimulation of the placental-gonadal axis. Maternal EGF levels increase just before fetal masculinization occurs.145 The placenta has an elevated concentration of EGF receptors, and placental stimulation by EGF may stimulate hCG production, which may also stimulate fetal Leydig cells to produce androgens that, alone or combined with other factors, may determine testicular descent. In contrast, estrogens play a negative role in testicular descent by preventing regression of the cranial gonadal ligament, gubernaculum growth, and fetal Leydig cell proliferation and thus causing a decrease in androgen and INSL3 secretion.147–151 Exposure to environmental endocrine disrupters in utero has a negative effect on male genital tract development. Estrogens are among those negative factors. During the first trimester of gestation, mothers of cryptorchid infants have free estradiol serum concentrations that are significantly higher than those of controls.147 Experimental studies have shown that estradiol diminishes gubernaculum swelling and stabilizes müllerian ducts; it has therefore been proposed that estradiol inhibits the cell proliferation that causes this swelling,149,150 through a reduction of INSL3 secretion by Leydig cell damage152 (Fig. 12-12). Fig. 12-12 Main factors involved in testicular descent. CGRP, Calcitonin gene–related peptide; CSL, cranial suspensory ligament; G, gubernaculum; INSL3, insulin-like factor 3; LGR8, leucine-rich repeat-containing G-protein–coupled receptor 8; T, testis. After birth, the gubernaculum and processus vaginalis undergo involution. The gubernaculum is replaced by fibrous tissue that forms the scrotal ligament. Once the testis has descended, the processus vaginalis undergoes atrophy and reabsorption, mainly in its cephalic portion. It has been suggested that a failure in the disappearance of the processus vaginalis could be a common cause of acquired cryptorchidism.153 In some patients, a noticeable and wide processus vaginalis is associated with inguinal hernia and cryptorchidism, whereas a narrow processus vaginalis appears associated with hydrocele; if there is partial obliteration of the lumen with persistence of the processus vaginalis, the testis could be retractile.154 From birth to puberty the testis is a dynamic structure. This is an important consideration when interpreting biopsy results in children. All testicular components undergo waves of proliferation and differentiation prior to puberty.155,156 The use of morphometric analyses based in proven stereologic methods, together with the performance of endocrinologic studies in infants and children, demonstrated that the numbers of both Sertoli cells and germ cells increase during this period, and significant production of AMH and inhibin occurs.157,158 The newborn testis has a volume of approximately 0.57 mL,159 and it is covered by a thin tunica albuginea from which the intratesticular septa arise. These septa divide the testis into approximately 250 lobules containing the seminiferous tubules and testicular interstitium (Fig. 12-13). The seminiferous tubules measure 60 to 65 µm in diameter, are solid cords, with no apparent lumina, and are surrounded by a thin basement membrane and isolated myoid cells and fibroblasts. The seminiferous tubules are filled with Sertoli cells and germ cells. Fig. 12-13 Longitudinal section of the testis and the head and tail of the epididymis from a newborn. Intratesticular septa split the testis into lobules that converge in the testicular mediastinum. Sertoli cells are the most abundant, with 26 to 28 cells per tubular cross section (Fig. 12-14). They form a pseudostratified cellular layer and have elongated to oval nuclei with darker chromatin than that of mature Sertoli cells, as well as one or two small peripheral nucleoli. The apical cytoplasm contains abundant rough endoplasmic reticulum, several Golgi complexes, and numerous vimentin filaments, and it expresses inhibin B (Fig. 12-15). Extended interdigitations and small junctions of the occludens and adherens types join adjacent Sertoli cells, and desmosome-like junctions are present between Sertoli cells and germ cells. Mitoses are occasionally seen. With immunohistochemical methods these cells express inhibin, AMH, and vimentin, and they also express M2A oncofetal antigen slightly.160 In the apical pole of Sertoli cells, spherical or ovoid bodies show intense immunostaining with inhibin161 (Fig. 12-16). Fig. 12-14 The seminiferous tubules contain Sertoli cells, which are the most numerous cells, as well as two germ cell types: gonocytes and spermatogonia. The gonocytes have large nuclei with large central nucleoli. The spermatogonia have smaller nuclei and pale cytoplasm. Several Leydig cells are seen in the interstitium. Fig. 12-15 Newborn testis. Both Sertoli cells and Leydig cells are intensely immunoreactive for inhibin. Fig. 12-16 Newborn testis. Inhibin inclusion in the apical pole of Sertoli cells. The clear unstained spaces correspond to germ cells. The germ cells comprise fetal spermatogonia, spermatogonia A dark (Ad), and gonocytes. Gonocytes are usually located near the center of the tubules, with spherical and voluminous nuclei and large central nucleoli.162 Most gonocytes immunostain with PLAP and c-kit. Spermatogonia are mainly located on the basal lamina in a discontinuous pattern, and they possess smaller nuclei and less cytoplasm than gonocytes; the nucleoli are peripheral and very small. At birth, most spermatogonia correspond to the adult type A (see the later discussion of the adult testis) (Fig. 12-17). Spermatogonia Ad have smaller nuclei, and barely visible nucleoli are peripherally distributed. Fig. 12-17 Spermatogonia show wide cytoplasm and regularly outlined nuclei with eccentric nucleoli. The cytoplasm contains mitochondria joined by electron-dense bars. Seminiferous tubules are surrounded by the tunica propria, which comprises a basal lamina, myoid cells, fibroblasts, collagen fibers, and extracellular matrix. The peritubular (myoid) cells express intense nuclear immunostaining for the AR, with expression similar to that of interstitial cells; this contrasts with negative staining in Sertoli cell nuclei.158 The testicular interstitium is a loose connective tissue that contains fetal Leydig cells that resemble adult Leydig cells but lack Reinke crystalloids163,164 (Fig. 12-18). These cells have well-developed smooth and rough endoplasmic reticulum, filament bundles, and lipid droplets. Additionally, mast cells, macrophages,165 and hematopoietic cells are present. The first important postnatal development of the testis occurs in the neonatal period and is known as minipuberty. It involves changes in germ cells, Sertoli cells, and Leydig cells. These changes are caused by a transient increase in secretion of both FSH and LH during the third postnatal month.166–172 Testicular weight and volume increase twofold from birth up to 5 months of age.173–175 FSH induces Sertoli cell proliferation.176 The number of these cells increases five to six times during the first year of life.177,178 Inhibin B, a Sertoli cell marker, also increases in this period, and its level remains elevated even when both FSH and LH levels have decreased.172 Under LH action, Leydig cells, which had initiated involution, undergo stimulation: their number increases, the organelles involved in steroid biosynthesis develop in these cells, and new Leydig cells begin to differentiate from peritubular myoid cells.164,179 Serum testosterone levels also increase.179 The reason for this increase could be either gonadotropic stimulation, which results from the elimination of placental steroids in newborn blood, or simple adaptation of the hypothalamohypophyseal-gonadal axis to the rise of sex hormone–binding globulin (SHBG) levels.180,181 The total number of germ cells per testis increases up to threefold in the first months of life to the end of this neonatal period,182 and it drops later.162 Gonocytes move from the center of the seminiferous tubule toward the basal lamina. This migration is probably facilitated by some cell adhesion molecules of the immature Sertoli cell surface, including P-cadherin.183 Transformation of gonocytes into spermatogonia Ad is enhanced by testosterone and probably also by AMH which is found at high levels between the fourth and twelfth months of life46 (Fig. 12-19). This transformation is completed at the age of 6 months and coincides with the loss of fetal germ cell markers (PLAP and c-kit). Results of these markers should be negative after the first year of age. Paraganglia are often observed in epididymides and spermatic cords from newborns. This finding is not surprising because paraganglia are the main source of catecholamine before birth184 (Fig. 12-20). From the sixth month to approximately the second half of the third year of life, the testis is in a resting period. The tubular diameter decreases (from 80 to 60 µm), and spermatogonia proliferation is hardly observed. Leydig cells are involuting, and at the end of this period only some of these cells persist, so they are not easily detected in routine slides. The albuginea thickness diminishes to 250 µm. Despite these findings, which permit investigators to define a resting period of the testis, Sertoli cells maintain active hormone synthesis. During these years Sertoli cells produce high levels of AMH185,186 and inhibin.172 AMH modulates the number and function of Leydig cells, by hindering the differentiation of these cells from their mesenchymal precursors, and the synthesis of steroidogenic enzymes.187 Inhibin B plays a role in the inhibition of FSH during infancy. Immunohistochemically, inhibin B expression is observed all over the cytoplasm and in a granular pattern in the apical pole. This quiescence is broken at the end of the third year by the second wave of germ cell proliferation.159 The testis enters a growth period. The number of Ap spermatogonia increases, and B spermatogonia (derived from Ap spermatogonia) appear. In some normal testes from children who are older than 4 years, meiotic primary spermatocytes and round spermatids (Sa + Sb types) are observed188 (Fig. 12-21). This second spermatogenic attempt fails, and many degenerate germ cells may be present but are phagocytosed by Sertoli cells.189,190 The testis continues to produce AMH (by Sertoli cells)185 and inhibin B.172 AMH modulates the number and function of Leydig cells by regulating differentiation of their mesenchymal precursors and the expression of steroidogenic enzymes.187 Inhibin B plays a role in FSH inactivation during infancy. Fig. 12-21 Testis from a 4-year-old child. The seminiferous tubules have spermatogonial proliferation and contain a central group of primary spermatocytes. The cause of this second wave of germ cell proliferation is unknown; no elevation of FSH or LH serum concentrations occurs between 6 months and 10 years of life. After the sixth year, there is a slight increase in adrenal androgens, but testicular testosterone levels increase only after the tenth year.191,192 By the third year, most Leydig cells have degenerated: from a peak of approximately 18 million at birth, only 60,000 remain by the age of 6 years. At this age, testosterone levels are similar to those of girls,191 and most androgens are of adrenal origin. Testosterone measurements performed during infancy have revealed that testosterone levels are much higher in the tunica vaginalis than in plasma.193 It also could be important that Sertoli cells begin to express the AR in their nuclei just at this age158 (Fig. 12-22). This expression is probably related to the development of this wave of proliferation and differentiation of germ cells.
Nonneoplastic diseases of the testis
Embryology and anatomy of the testis
Embryology
Development of the testis
Genetic mechanisms involved in sex determination and testicular differentiation
Development of the bipotential gonad
Formation of the gonadal ridge.
Primordial germ cells: origin, migration, and formation of the gonadal blastema.
Male-female determination
Testis differentiation: development of seminiferous cords and interstitium
Early organization of the gonadal blastema.
Differentiation of primordial germ cells.
Sertoli cell differentiation.
Peritubular myoid cell differentiation.
Leydig cell development.
Rete testis formation.
Development of the urogenital tract
Hormonal control of male genital tract differentiation
Fetal testis structure
Supporting structures
Seminiferous cords
Germ cells
Sertoli cells
Peritubular myoid cells
Leydig cells
Vascularization of the fetal testis
Fetal epididymis
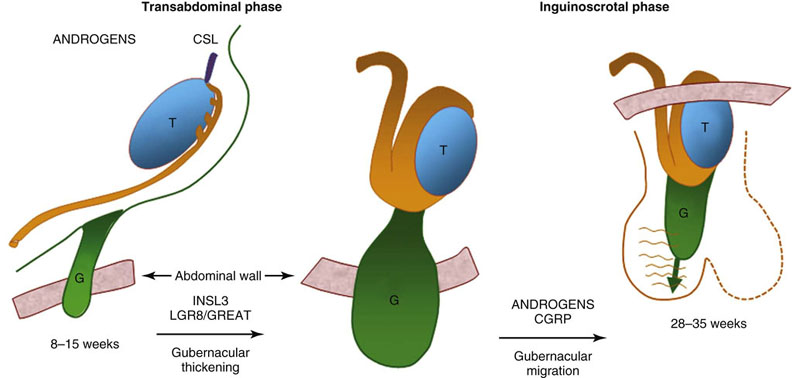
Testicular descent
Anatomic structures involved in the testicular descent
Prerequisites for testicular descent
Normal function of the hypothalamopituitary-testicular axis.
Adequate intraabdominal pressure.
Adequate development of the processus vaginalis.
Factors that regulate testicular descent
Prepubertal testis
Development of the testis from birth to puberty
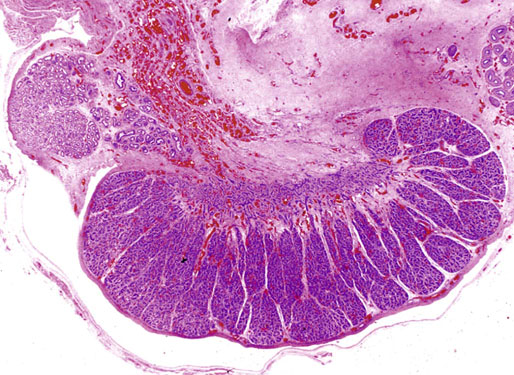
The testis at birth
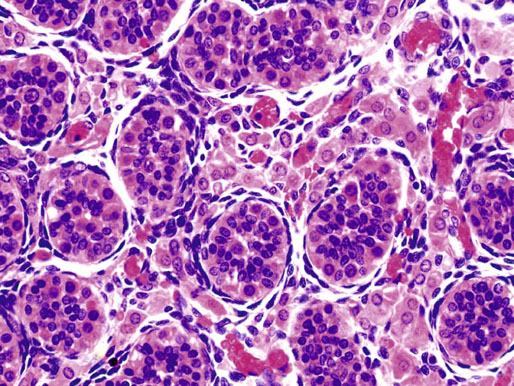
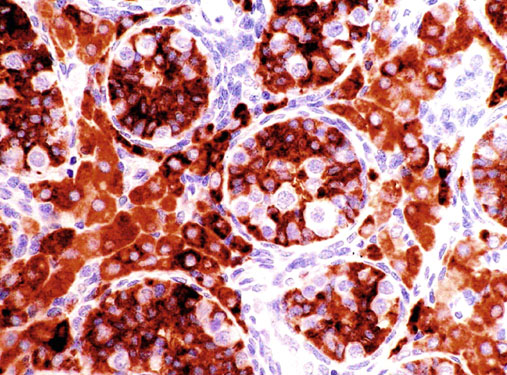
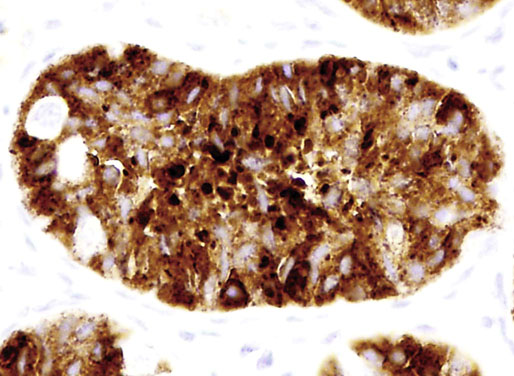
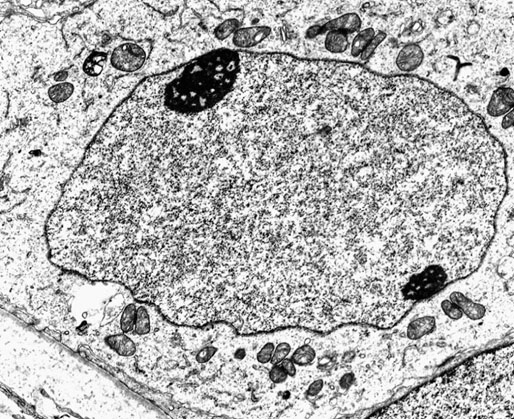
Neonatal development of the testis
Testis in infancy