Chapter 70B Nonhepatic surgery in the cirrhotic patient
Overview
Chronic liver disease and cirrhosis were the twelfth leading cause of death in the United States and resulted in more than 30,000 deaths in 2009 (Kochanek et al, 2011). Chronic viral infection and alcohol abuse account for the majority of the disease burden globally, but the incidence of obesity-associated nonalcoholic fatty liver disease accounts for an ever-increasing proportion of cases, especially in Western societies. Furthermore, clinicians continue to gain knowledge and skills to care for patients with cirrhosis in the end stages of their disease. These facts explain why a significant increase has been seen in the number of patients with comorbid liver disease and cirrhosis encountered in both general and specialty surgical practice.
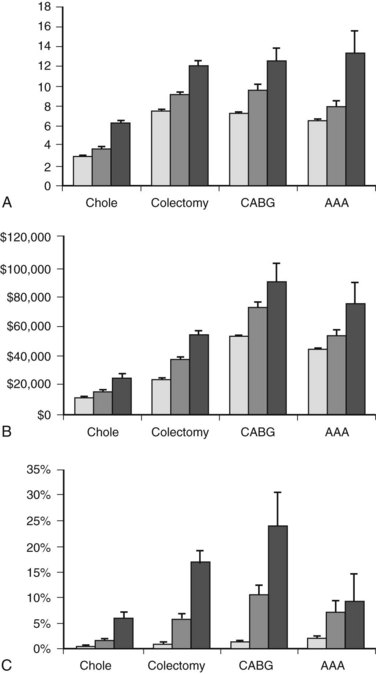
Cirrhosis can have dramatic effects on multiple organ systems, making surgery on the cirrhotic patient a complex and difficult undertaking. A recent, large, population-based study demonstrated that people with cirrhosis, in particular those with portal hypertension, have significantly worse outcomes after elective operations than those without (Fig. 70B.1; Csikesz et al, 2009). The mere act of opening the abdominal wall in a cirrhotic patient with portal hypertension causes collateral blood vessels to dilate and may lead to systemic hypotension and hepatic decompensation secondary to ischemia (Haskal et al, 1994; Norton et al, 2003).
Evaluation and Stratification of Liver Disease
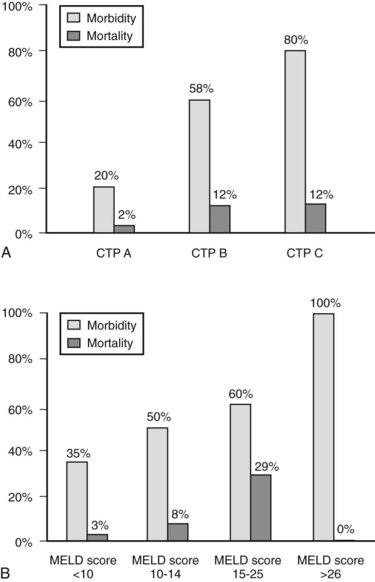
The decision regarding whether a cirrhotic patient is medically fit to undergo an operation can be difficult to make. Many factors must be considered, but the most important are the magnitude and necessity of the proposed operation, the nonhepatic comorbidities of the patient, and the severity of the liver disease. Numerous factors have been correlated with poor outcome in patients with cirrhosis—including low albumin levels, blood transfusion requirements, abnormal coagulation, and ascites—and various scoring systems to gauge these have evolved. The first, most widely used scoring system is the Child-Turcotte-Pugh (CTP) system, which incorporates several objective and subjective variables to stratify severity of liver disease. Historically, patients with CTP scores of A, B, or C have been shown to have operative mortality rates of 10%, 30%, and 80%, respectively (Garrison et al, 1984; Mansour et al, 1997).
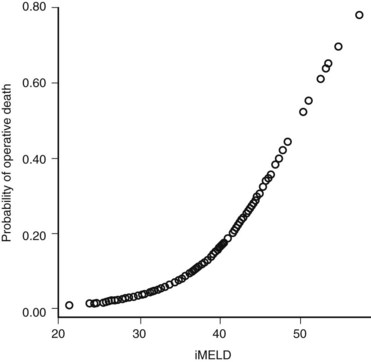
The Model for End-Stage Liver Disease (MELD) score was developed to predict death after transjugular intrahepatic portosystemic shunt (TIPS) in an effort to determine who was likely to progress toward needing liver transplantation. In an effort to improve on the tripartite CTP system, researchers have recently evaluated to ability of MELD to predict morbidity and death after nonshunt abdominal surgery (Befeler et al, 2005; Farnsworth et al, 2004). As with CTP, MELD correlates with risk of postoperative death (Figs. 70B.1 and 70B.3). Several reports have found that MELD is superior to CTP in predicting postoperative problems, although these improvements generally are modest (Perkins et al, 2004). Interestingly, the survival rates from the more recent series indicate a fairly dramatic improvement in survival compared with older series (Fig. 70B.3).
In addition to factors considered in MELD and CTP scoring, other factors have been identified for risk stratification and prediction in cirrhotic patients undergoing major operations. These include elevated creatinine level, chronic obstructive pulmonary disease (COPD), male gender, and an American Society of Anesthesiologists (ASA) class of IV or V (Ziser et al, 1999). Teh and colleagues (2007) demonstrated ASA classification as a useful marker to further stratify the comorbid illness in cirrhotic patients preoperatively. This case-control study of cirrhotic patients who underwent major nontransplant operations identified MELD score, ASA class, and age as predictors of perioperative death. An ASA class of IV was equivalent to the addition of 5.5 MELD points in added risk, and age greater than 70 years was equivalent to three additional MELD points. A single point increase in MELD score was associated with a 15% increase in perioperative death. Emergency surgery predicted in-hospital death, although patients undergoing emergency operations had a higher median MELD score. ASA class V was the strongest predictor of 7-day mortality, and MELD score was the most robust predictor beyond 7 days. The median survival of patients in this series was 4.8 years for a MELD score of 0 to 7, 3.4 years for a score of 8 to 11, 1.6 years for 12 to 15, 64 days for 16 to 20, 23 days for 21 to 25, and 14 days for 26 or greater.
Perioperative Management and Optimization
Most cirrhotic patients have protein-energy malnutrition, placing them at an increased risk for developing a variety of postoperative complications, such as wound dehiscence, infections, reaccumulation of ascites, and death (Merli et al, 2002). Nutritional status should be evaluated preoperatively; when found to be deficient, efforts should be undertaken to improve it (see Chapter 24). Nasoenteric or parenteral feedings that use low-sodium formulas supplemented with trace elements and branched-chain amino acids are an option for patients who are unable to adequately nourish themselves.
Coagulopathy secondary to impaired hepatic synthesis of clotting factors and decreased vitamin K stores as a result of malnutrition and poor intestinal absorption may be present. Thrombocytopenia is also common in these patients secondary to splenic sequestration and bone marrow suppression. Preoperative transfusions of fresh frozen plasma and platelets lower the risk of bleeding. In the operating room, careful tissue handling and the maintenance of a low central venous pressure are other factors that can help to minimize blood loss (Alkozai et al, 2009). If significant bleeding arises, cryoprecipitate, diamino-8-d-arginine vasopressin, and recombinant factor VIIa are additional options to reverse coagulopathy and control hemorrhage (O’Leary et al, 2009).
In the perioperative period, altered mental status in a cirrhotic patient should be thoroughly evaluated. Only after all other potential causes have been ruled out can hepatic encephalopathy be diagnosed. With the acute onset of encephalopathy, protein intake may be briefly withheld. Lactulose should be administered and titrated for three soft bowel movements a day. Oral antibiotics, such as metronidazole or neomycin, may also be used to decrease the intestinal bacterial load (Muilenburg et al, 2009). Surgery should be postponed if encephalopathy arises preoperatively. Postoperatively, the administration of narcotics and other sedating medications should be kept to a minimum. There is no role for prophylactic lactulose therapy for all cirrhotic patients undergoing surgery (O’Leary et al, 2009).
Perioperative fluid management in cirrhotic patients can be very difficult. Patients with cirrhosis should be adequately resuscitated to avoid hypotension and hepatic ischemia, but fluids should also be given judiciously to minimize the accumulation of ascites postoperatively. One center that performs a large volume of surgery on patients with end-stage liver disease limits the infusion of crystalloid solutions in the perioperative period (Telem et al, 2010b). Instead, all patients with advanced liver disease are placed on a postoperative sodium-poor albumin drip until they are able to resume oral intake.
Ascites increases the risk of postoperative renal failure, infections, and wound dehiscence. Even if ascites is drained at the time of surgery, it rapidly reaccumulates in the postoperative period. Medical therapy, which includes salt restriction and diuretics, is typically considered the first line of treatment. TIPS, however, may be considered a first-line treatment for refractory ascites and may be placed before elective surgery to help control ascites throughout the entire perioperative period (Azoulay et al, 2001; Gines et al, 2004; Schlenker et al, 2009). Moreover, TIPS may also reduce the risk of significant perioperative bleeding. In one of the largest series on the use of TIPS in patients undergoing major extrahepatic surgery, 25 cirrhotic patients with a mean MELD score of 15 ± 7.6 underwent TIPS at a median of 20 days before major abdominal and cardiothoracic operations. Blood transfusions were relatively minimal in the series, with a median of 3 units for abdominal operations (range, 0 to 21) and 4 units for cardiothoracic operations (range, 0 to 20). With a median follow-up of 33 months, actuarial 1-year patient survival was 74%, and the three postoperative deaths in the series occurred in patients with MELD scores higher than 24, all of whom underwent emergent surgery (Kim et al, 2009). Studies that compare outcomes after surgery with preoperative TIPS placement versus those with no TIPS, however, are lacking. Also, the optimal time interval between TIPS placement and elective surgery is unclear, but it is common for a period of several weeks to be required to see a clinical improvement in ascitic volume after TIPS; therefore shunts should probably be placed several weeks prior to planned operations.
Specific Procedures
Cholecystectomy
The incidence of gallstones in patients with cirrhosis is twice that of the general population as a result of increased intravascular hemolysis and decreased gallbladder motility and emptying (Bouchier, 1969; Conte et al, 1991; Schwesinger et al, 1985). In the 1980s, rates of major morbidity and mortality in patients with cirrhosis undergoing cholecystectomy were as high as 35% and 25%, respectively (Aranha et al, 1982; Cryer et al, 1985; Garrison et al, 1984; Schwartz, 1981). Postoperative death occurred secondary to blood loss, sepsis, and liver failure. Almost invariably, these procedures were performed as open laparotomy. At that time, the presence of cirrhosis was considered a contraindication for the laparoscopic approach, based on the assumption that it would lead to increased rates bleeding and liver failure (Yerdel et al, 1993). However, as greater experience in laparoscopic surgery has been gained, this point of view has changed, and now many studies demonstrate favorable morbidity and minimal to no mortality in patients with early cirrhosis (CTP class A and B) undergoing laparoscopic cholecystectomy (Cucinotta et al, 2003; Fernandes et al, 2000; Ji et al, 2004; Morino et al, 2000; Poggio et al, 2000; Yeh et al, 2002). A recent meta-analysis comparing laparoscopic cholecystectomy in cirrhotic and noncirrhotic patients demonstrated significantly higher rates of morbidity (21% vs. 8%, respectively; P < .001), intraoperative bleeding (26% vs. 3%; P < .001), and open conversion (7% vs. 4%; P < .05), but no difference was reported in terms of wound infection and mortality rates between the two (Puggioni & Wong, 2003).
Laparoscopic cholecystectomy offers improved visualization for meticulous dissection and avoids the need for a large subcostal or upper midline incision. It is associated with less operative blood loss, shorter operative time, and decreased length of hospital stay versus the open procedure in cirrhotic patients (Puggioni & Wong, 2003). Furthermore, laparoscopic cholecystectomy leads to fewer adhesions in the right upper quadrant, which are associated with increased bleeding complications and longer operative times in patients who ultimately undergo liver transplant.
Some specific technical considerations must be considered when performing laparoscopic cholecystectomy in a cirrhotic patient. In placing the umbilical port, particular care must be taken to avoid venous collaterals and the umbilical vein. One approach may be to place one of the other ports first, such as the subxiphoid port, in order to then place the umbilical port under direct visualization. Transillumination of the abdominal wall helps the surgeon to avoid these and other venous collaterals during trocar placement. Furthermore, the subxiphoid port should be placed off midline to avoid the falciform ligament and the recanalized umbilical vein. Careful traction on the gallbladder helps to avoid undue bleeding as it is dissected off the liver. Instruments such as the Harmonic Scalpel (Ethicon, Somerville, NJ) or LigaSure (Covidien, Mansfield, MA) may also be used to help control bleeding during the dissection (Curro et al, 2005; El-Awadi et al, 2009; Morino et al, 2000; Schiff et al, 2005; Tuech et al, 2002). A subtotal or supracystic cholecystectomy, leaving part of the gallbladder wall attached to the liver bed and ligating the cystic duct from within the gallbladder with a purse-string suture, is an option in patients with large pericholecystic venous collaterals (Bornman & Terblanche, 1985; see Chapter 33). Trocars should also be removed under direct visualization to ensure adequate abdominal wall hemostasis at the end of the procedure. Finally, if anatomy is unclear or any doubt exists as to the safety of continuing laparoscopically, the procedure should be converted to open.
In patients with advanced cirrhosis (CTP class C), cholecystectomy in general is associated with very poor outcomes. Despite the lack of controlled trials, the literature supports the use of alternatives to cholecystectomy, such as a percutaneous cholecystostomy, in this high-risk group (Cucinotta et al, 2003; Curro et al, 2005; Delis et al, 2010; Yeh et al, 2002). An endoscopically placed cystic duct stent is another option proposed by some (Gaglio et al, 1996; Shrestha et al, 1996, 1999).
In general, the CTP scoring system is used in the literature to stratify patients suitable for cholecystectomy. Although some evidence suggests that the MELD classification system may be more accurate in predicting postoperative complications, these data are inconsistent, and cutoff values vary among studies (Delis et al, 2010; Perkins et al, 2004). Therefore, until the MELD system has been further validated, CTP should be used for stratification. In CTP classes A and B patients with symptomatic cholelithiasis and/or acute cholecystitis, laparoscopic cholecystectomy should be performed by surgeons experienced in managing cirrhosis. In patients with class C cirrhosis, surgery should be deferred until liver disease is under better control, and alternate interventions, such as percutaneous cholecystostomy or endoscopic stenting, should be used.
Herniorrhaphy
Umbilical hernias occur in up to 20% of patients with cirrhosis. The pathogenesis of umbilical hernias in the setting of cirrhosis is multifactorial; ascites causes increased intraabdominal pressure, poor nutritional status leads to decreased abdominal muscle mass and fascial strength, and umbilical vein dilation results in enlargement of the preexisting supraumbilical fascial opening (Belghiti & Durand, 1997). In addition to the risks of bowel incarceration and strangulation that affect all patients with umbilical hernias, additional unique risks accompany having an umbilical hernia and cirrhosis. Skin ulceration over the hernia may be associated with the leakage of ascites, sac rupture, bacterial peritonitis, and even evisceration, with very high attendant rates of morbidity and mortality. Although rare, spontaneous umbilical rupture is associated with up to a 60% perioperative mortality rate and has been shown to correlate independently with an adverse outcome after emergent repair (Telem et al, 2010a).
Historically, mortality rates for umbilical hernia repair (UHR) in patients with cirrhosis were prohibitively high, and the prevailing opinion among surgeons was that uncomplicated hernias should be left untreated (Baron, 1960; O’Hara et al, 1975). UHR was only undertaken when complications, such as incarceration, strangulation, skin breakdown or impending rupture, or overt rupture, necessitated immediate intervention. In the present era, however, outcomes appear to be significantly improved for elective and even complicated UHR in patients with end-stage liver disease. Most series published in the literature since the early 1980s have reported little to no perioperative deaths and relatively low morbidity, although most of them have been relatively small retrospective studies (Belghiti et al, 1990; de la Pena et al, 2000; Ozden et al, 1998; Pescovitz, 1984; Runyon & Juler, 1985).
Marsman and colleagues (2007) looked at outcomes of elective UHR versus conservative management in patients with umbilical hernias, cirrhosis, and ascites. Seventeen patients with a median MELD score of 23 (range, 18 to 26) underwent elective UHR, and another 13 patients, also with a median MELD score of 23 (range, 18 to 27), were observed. In the elective UHR group, 3 patients (18%) had local wound complications, but there were no instances of hepatic decompensation or perioperative death. In contrast, in the group that was observed, 10 patients (77%) developed complications, including nine cases of incarceration and one spontaneous rupture with evisceration; these led to emergency UHR in six patients, and there were two perioperative deaths (15%). Although this was a retrospective study, and it is likely that some selection bias existed between the two groups, it is one of the few studies to compare early UHR versus watchful waiting in patients with comparable and relatively high MELD scores.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree