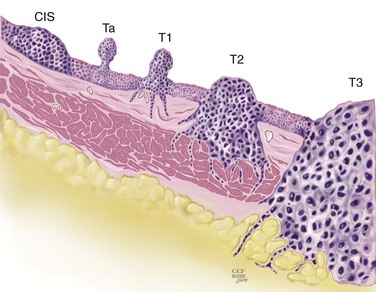
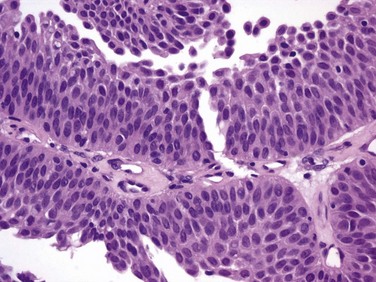
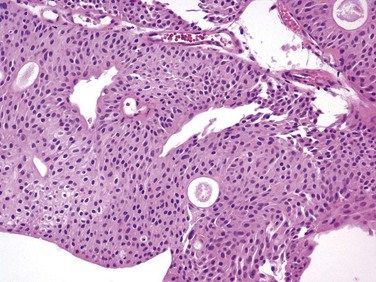
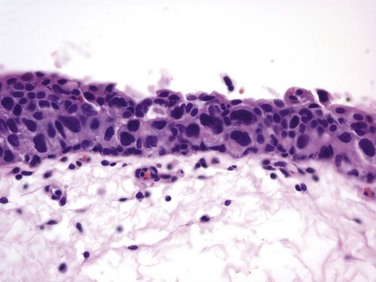
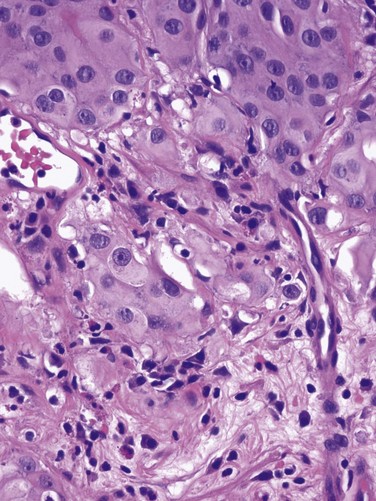
J. Stephen Jones, MD, MBA, William A. Larchian, MD Traditionally known as superficial bladder cancer, malignant urothelial tumors that have not invaded the detrusor are more appropriately termed non–muscle invasive (Epstein et al, 1998; Smith et al, 1999). The former term suggested that all such tumors shared the relatively benign course of low-grade papillary tumors, giving misleading reassurance to patients with the highly malignant subcategories of carcinoma in situ (CIS) and high-grade Ta and T1 lesions. Approximately 70% are non–muscle invasive at presentation. Of these, 70% present as stage Ta, 20% as T1, and 10% as CIS (Fig. 81–1) (Ro et al, 1992). The presence of bladder cancer is usually suspected by hematuria. Patients with macroscopic (gross) hematuria have reported rates of bladder cancer of 13% to 34.5% (Lee and Davis, 1953; Varkarakis et al, 1974). Microscopic hematuria is associated with a 0.5% to 10.5% rate of bladder cancer (Golin and Howard, 1980; Mohr et al, 1986; Sultana et al, 1996; Khadra et al, 2000). The presence of irritative voiding symptoms may double the risk, especially for CIS (5% vs. 10.5%) (Mohr et al, 1986). The Mayo Clinic reported that 80% of patients with CIS presented with irritative symptoms (Zincke et al, 1985). In a review of 600 patients diagnosed with interstitial cystitis, 1% of the patients had a missed diagnosis of urothelial carcinoma. Of note, two thirds of these patients did not have hematuria (Tissot et al, 2004). Thus cystoscopy and upper tract imaging are indicated in patients with hematuria and/or unexplained irritative symptoms (Grossfeld et al, 2001). Recurrence is common in all patients with non–muscle-invasive urothelial cancer but can often be controlled successfully with transurethral surgery, intravesical therapy, or a combination. In contrast to recurrence, patients can be divided into low or high risk for progression, which is the true concern. Low-grade Ta lesions are low risk, whereas all high-grade lesions (including CIS) have a high risk of progressing. The frequency of progression and death is shown in Table 81–1. Table 81–1 Estimates of Disease Progression in Non–Muscle-Invasive Bladder Cancer: World Health Organization/International Society of Urologic Pathology Consensus Classification In contrast, essentially benign papillary tumors with orderly cellular arrangement, minimal architectural abnormalities, and minimal nuclear atypia are distinct from those two grades and are designated Papillary Urothelial Neoplasm of Low Malignant Potential (PUNLMP). Such tumors would have been labeled either papillomas or grade 1 TCC in older systems but are now regarded as so unlikely to progress that they are considered benign. However, on the basis of this low risk, the WHO recommends that such pathology reports contain the note, “Patients with these tumors are at risk of developing new bladder tumors (“recurrence”), usually of similar histology. However, occasionally these subsequent lesions manifest as UC, such that follow-up of the patient is warranted” (Epstein et al, 1998). Papillomas are truly benign and not associated with risk of progression (Figs. 81-1 through 81-5). The bladder has three main histologic layers: (1) urothelium, (2) suburothelial loose connective tissue called lamina propria, and (3) detrusor or muscularis propria (which is absent beneath the urothelium of diverticulae). Stage Ta denotes a papillary tumor confined to the urothelium. CIS (also termed Tis) is a flat, high-grade lesion confined to the same layer, and T1 is a tumor invading lamina propria. The TNM grading system for non–muscle-invasive tumors is demonstrated in Figure 81–1. Deep lamina propria invasion carries a substantially more serious prognosis in some reports, and some have proposed these be subcategorized as T1b (Younes et al, 1990). However, the value of substaging has not been validated in other studies, so the 1998 Bladder Cancer Consensus Conference Committee rejected the concept (Platz et al, 1996; Epstein et al, 1998). Low-grade Ta lesions recur at a rate of 50% to 70% and progress in approximately 5% of cases. In contrast, high-grade T1 lesions recur in more than 80% of cases and progress in 50% of patients within 3 years. This behavior is primarily grade, rather than stage, dependent, because patients with high-grade tumors progressed with similar frequency regardless of whether they were invasive (T1) or noninvasive (Ta) (Herr, 2000a). Prognosis also correlates with tumor size, multiplicity, papillary versus sessile configuration, presence or absence of lymphovascular invasion, and status of the remaining urothelium (Althausen et al, 1976; Lutzeyer et al, 1982; Heney et al, 1983a, 1983b; Kunju et al, 2008). The variance in biologic behavior for low-grade versus high-grade lesions correlates with the known dual molecular lines of genetic development for these two pathways and supports the concept that high-grade and low-grade cancers may be considered as essentially different diseases (Hasui et al, 1994; Droller, 2005). Chromosomal alterations caused by oxidative DNA damage create two separate genetic pathways to the development of UC (Spruck et al, 1994; Richter et al, 1997; Cote and Chatterjee, 1999). The first and more common (low grade) leads to noninvasive, papillary tumors. These usually follow an indolent course unless they convert to or are associated with a tumor of the second pathway (Kiemeney et al, 1993). The second pathway leads to the development of high-grade cancer including CIS, T1, and, ultimately, muscle-invasive carcinoma. Such genetic alterations can be evaluated using karyotyping, microsatellite analysis for allelic imbalance (Mao et al, 1996), comparative genomic hybridization (Kallioniemi et al, 1995), DNA ploidy analysis by flow cytometry (Bittard et al, 1996), and fluorescence in-situ hybridization (FISH) (Degtyar et al, 2004). These evaluations can show that low-grade papillary tumors tend to exhibit relatively few chromosomal abnormalities, primarily involving loss of all or part of chromosome 9 (particularly the q arm). In contrast, high-grade tumors tend to have numerous and greatly variable chromosomal gains and losses. In addition to their relatively predictable aneuploidy, high-grade tumors can also lose all or part of chromosome 9 (Richter et al, 1997). Although almost any chromosome can be affected, aneuploidy of chromosomes 7, 9, and 17 is associated with especially aggressive tumors (Olumi et al, 1990; Waldman et al, 1991; Degtyar et al, 2004). Because of these differing genetic imprints, it has been suggested that papillary pTa tumors could almost be considered benign and might be a completely separate disease entity in contrast to high-grade tumors (Sauter and Mihatsch, 1998; Harnden, 2007). Nevertheless, high-grade and low-grade lesions are known to coexist. UC is traditionally considered a field change disease, with tumors arising at different times and sites. Rarely, patients who initially have low-grade tumors will subsequently develop high-grade tumors, often years after the original tumors, so long-term surveillance is usually reasonable (Prout and Barton, 1992). Stage Ta tumors are usually low grade. Although recurrence is common, especially in the setting of multiplicity, progression is rare. However, 2.9% to 18% of Ta tumors are high grade, with an average of 6.9% (Sylvester et al, 2005). The most important risk factor for progression is grade, not stage (Norming et al, 1992; Millan-Rodriguez et al, 2000). Stein and colleagues (2001) found little difference in survival comparing noninvasive and muscle-invasive tumors as long as the cancer was organ confined (Stein et al, 2001; Sylvester et al, 2005). CIS is occasionally mischaracterized as “premalignant” (Sylvester et al, 2005), but it is actually a flat, noninvasive UC that is high grade by definition. Although confined to the urothelium in the same manner as stage Ta, CIS is regarded as a precursor for the development of invasive high-grade cancer. CIS lesions are composed of severely dysplastic urothelium. Microscopically, the slide will demonstrate disorderly histology with nuclear atypia characteristic of high-grade malignancy; denudement of some or all of the mucosa due to loss of cellular cohesion sometimes complicates interpretation. A pathology report read as dysplasia or atypia can create confusion. Most pathologists consider mild examples of these entities to be benign. However, lesions interpreted as severe dysplasia or severe atypia are regarded as being the same entity as CIS (Epstein et al, 1998). Again, unambiguous communication between pathologist and urologist can minimize the risk for misinterpretation. Between 40% and 83% of patients with CIS will develop muscle invasion if untreated, especially if associated with papillary tumors (Althausen et al, 1976). Among patients thought to have CIS alone, up to 20% who are treated with cystectomy are found to contain invasion on final pathology (Farrow et al, 1976). The presence of CIS in cystectomy specimens performed for presumed T1 tumors was associated with upstaging in 55% of patients in a recent series, compared with 6% upstaging in patients without CIS (Masood et al, 2004). In a series of 1500 patients, CIS was the second most important prognostic factor after grade (Millan-Rodriguez et al, 2000). Multicentricity presents another ominous characteristic of CIS (Koch and Smith, 1996). The presence of irritative voiding symptoms has been associated with diffuse disease, invasion, and a compromised prognosis, but there is no consensus on this finding in the literature (Smith et al, 1999; Sylvester et al, 2005). T1 tumors are usually papillary; a nodular or sessile appearance suggests deeper invasion. Deep penetration into the lamina propria, especially if involving muscularis mucosae, increases the risk of recurrence and progression in some reports. Lymphovascular invasion increases the risk as well (Lotan et al, 2005). Hydronephrosis usually indicates muscle invasion. There is significant potential for understaging in patients with high-grade, apparently non–muscle-invasive tumors, especially for those that appear to be stage T1. Many tumors are found to be more extensive than the transurethral resection (TUR) specimen indicated when patients undergo cystectomy. Stein (Freeman et al, 1995) reported that one third of patients believed to have non–muscle-invasive disease at the time of cystectomy were found to actually have muscle invasion, only half of which were organ confined. Metastases were already present in 8% of these patients (Freeman et al, 1995). Stein’s subsequent review noted that understaging errors from 34% to 62% have been reported (Stein, 2001), and a study from the Mayo Clinic before widespread use of intravesical therapy showed that 78% of patients with clinical T1 disease that underwent cystectomy had muscle invasion, with 62% having extravesical disease. Studies from the past decade addressing the risk of understaging of T1 tumors are shown in Table 81–2. Table 81–2 Risk of Understaging When Cystectomy Is Performed for Presumed Non–Muscle-Invasive Disease Key Points: Pathology Upper tract imaging is usually performed both to identify other sources of hematuria and to assess the extravesical urothelium due to the “field change” nature of UC that can affect such cells throughout the urinary tract. Expert consensus is that patients with solitary or limited low-grade Ta lesions do not need imaging, owing to the very low risk of extravesical disease (Goessl et al, 1997). Complete visualization to plan the resection is facilitated by either the flexible cystoscope or preferably the 70-degree rod lens, which allows maintenance of the anatomic relationships. Resection is performed using a 30-degree lens placed through a resectoscope sheath because this deflection allows visualization of the loop placed at this location. Continuous irrigation with the bladder filled only enough to visualize its contents minimizes bladder wall movement and lessens thinning of the detrusor through overdistention (Koch and Smith, 1996). Video TUR allows magnification, facilitates resident teaching, allows documentation of findings, and reduces the risk of body fluid exposure to the surgeon (Manoharan and Soloway, 2005; Nieder et al, 2005). Resection is performed piecemeal, delaying transection of any stalk until most tumor is resected in order to maintain countertraction. Friable, low-grade tumors can often be removed without the use of electrical energy because the nonpowered cutting loop will break off most segments. This minimizes the chance of bladder perforation. Higher-grade, more solid tumors and the base of all tumors require the use of cutting current; cautery yields hemostasis once all of the tumor is resected. Lifting the tumor edge away from detrusor lessens the chance of perforation (Holzbeierlein and Smith, 2000). Repeated slow fulguration may complicate the ability of the pathologist to determine grade or invasion status. Traditionally, TUR has been performed in sterile water because saline solutions conduct electricity and disperse energy from the monopolar cautery cutting loop. Glycine is more expensive, and there is no evidence of its benefit compared with water (Holzbeierlein and Smith, 2000). Introduction of bipolar electroresection is reported to allow transurethral resection in saline (TURIS) and to minimize the risk of the obturator reflex that can predispose to bladder perforation (Shiozawa et al, 2002; Miki et al, 2003). The use of general anesthesia with muscle-paralyzing agents also prevents obturator reflex. This can also be accomplished by direct injection of 20 to 30 mL of local anesthetic (lidocaine) into the obturator nerve and its canal, but few centers have experience with this. Care must be taken during resection near the ureteral orifice to prevent obstruction from scarring after fulguration. Pure cutting current causes minimal scarring and may be safely performed including resection of the orifice if necessary. Resection of the intramural ureter can lead to complete eradication of the tumor but risks reflux of malignant cells. The clinical implications of this are unclear (Palou et al, 1992). The vast majority of perforations are extraperitoneal, but intraperitoneal rupture is possible when resecting tumors at the dome (Collado et al, 2000). The risk of tumor seeding due to perforation appears to be low (Balbay et al, 2005). Anecdotal reports have identified extravesical recurrences after perforation, theoretically due to seeding (Mydlo et al, 1999). It has been suggested that the risk of tumor seeding is higher in patients who undergo surgical repair, but this may be related to patient selection because only serious intraperitoneal perforations are likely to be managed in this manner (Mydlo et al, 1999; Skolarikos et al, 2005). TUR syndrome from fluid absorption is uncommon and managed in the same manner as during TURP. As long as resection of the ureteral orifice is done with pure cutting current, scarring is minimal and obstruction unlikely. Cystoscopy to visualize efflux, which is occasionally aided by intravenous administration of indigo carmine or methylene blue or retrograde ureteropyelography, can determine presence or absence of obstruction. Balloon dilation of the orifice or endoscopic incision can relieve obstruction, but failure to respond may require reimplantation (Chang et al, 1989). Complete tumor removal is not always possible, whether due to excessive tumor volume, anatomic inaccessibility, medical instability requiring premature cessation, or risk of perforation. However, even in the absence of these circumstances repeat TUR is often indicated. When repeat TUR is performed within several days to several weeks of the original resection, residual tumor is identified at the site of the initial resection at least 40% of the time (Klan et al, 1991; Mersdorf et al, 1998; Vogeli et al, 1998). In a review, Miladi and coworkers (2003) found that a second TURBT performed within 6 weeks of the initial resection detected residual tumor in 26% to 83% of cases and corrected clinical staging errors in half of those cases. The potential for understaging high-risk disease ranged from 18% to 37% (Amling et al, 1994). Repeat TURBT is usually appropriate in the evaluation of T1 tumors because a repeat TUR can demonstrate worse prognostic findings in up to 25% of specimens (Schwaibold et al, 2000). This is especially likely if no muscle is identified on initial pathology, which can occur in almost half of cases. The Vanderbilt University group reported a 64% risk of understaging T1 lesions when muscle was absent, compared with 30% when muscle was present in the specimen (Dutta et al, 2001). Herr (1999) reported that a second resection changed treatment in one third of cases. Importantly, survival was 63% in patients who underwent a second TURBT versus 40% for those who did not in a German observational study (Grimm et al, 2003). The efficacy of bacillus Calmette-Guérin (BCG) in preventing progression appears to be higher in patients with high-grade papillary tumors and CIS if a restaging TURBT was performed before instillation of BCG (Herr, 2005). There appears to be variability in completeness of resection among surgeons. In patients with multiple tumors who had adjuvant treatment, recurrence rates varied between 7.4% and 45.8% depending on the surgeon (Brausi et al, 2002). Repeat resection is helpful in the setting of a second opinion unless clear evidence of muscle invasion is identified on the initial resection, especially if the outside pathology slides are not available for review. Consensus is that patients with pT1 and high-grade Ta tumors merit repeat resection. There is no consensus on timing of repeat TURBT, but most authors recommend 1 to 6 weeks after the initial resection (Nieder et al, 2005). Biopsies of any suspicious areas are an important part of a complete evaluation. Cold-cup biopsies may not provide as much information regarding muscular invasion but provide tissue sampling without cautery artifact that can interfere with pathologic interpretation (Soloway et al, 1978; Smith, 1986a, 1986b). On the basis of the understanding that CIS can exist in normal-appearing urothelium, some authors advocate the use of random biopsies to identify CIS in otherwise normal-appearing mucosa. This remains controversial. May and colleagues (2003) performed random biopsies in high-risk patients and found that the results were positive in 12.4% and altered treatment in 7% including 14 of 1033 patients in whom the only positive tissue was in the random biopsy, not the primary resected tumor. However, even when velvety red patches were sampled, only 11.9% of biopsies were positive in one report (Swinn et al, 2004). A European Organisation for Research and Treatment of Cancer (EORTC) retrospective review found that 10% of random biopsies were positive (3.5% CIS) and concluded such biopsies were not warranted (van der Meijden et al, 1999). Fujimoto and colleagues (2003) prospectively evaluated the role of random biopsies of normal-appearing urothelium and found cancer in only 8 of 100 biopsy samples, 5 of which were CIS. They concluded that random biopsies are indicated only in the setting of multiple tumors or positive cytology. The current consensus is that random biopsies are not indicated in low-risk patients (i.e., those with low-grade papillary tumors and negative cytology). Prostatic urethral biopsy using the cutting loop may be performed, especially if neobladder creation is anticipated for high-risk disease, but bleeding may be more common (Holzbeierlein and Smith, 2000). The additional value of the information obtained from cold cup and urethral biopsies must be weighed against the theoretical risk that biopsies provide an exposed bed to aid tumor implantation (Mufti and Singh, 1992; Kiemeney et al, 1994; Yamada et al, 1996). Traditional teaching is that TUR of the prostate (TURP) and TURBT of a low-grade bladder tumor may be performed at the same setting but that resection of a high-grade bladder tumor should not be resected coincident to TURP to avoid tumor seeding and possible intravasation of tumor cells likely to metastasize. Despite anecdotal reports of low-grade tumors implanting in the prostatic urethra after simultaneous resection, this risk appears to be small and the literature does not adequately address the risk of metastasis in this setting (Tsivian et al, 2003). It is believed that tumor cell implantation immediately after resection is responsible for many early recurrences and this has been used to explain the observation that initial tumors are most commonly found on the floor and lower side walls of the bladder, whereas recurrences are often located near the dome (Heney et al, 1981). Thus intravesical chemotherapy to kill such cells before implantation has been used (Zincke et al, 1983; Klan et al, 1991). Mitomycin C (MMC) appears to be the most effective adjuvant intravesical chemotherapeutic agent perioperatively, although epirubicin is used in Europe and direct comparative studies are lacking (Witjes and Hendricksen, 2008). Consistent with its proposed mechanism of action to prevent tumor cell implantation, a single dose administered within 6 hours lessens recurrence rates, whereas a dose 24 hours later does not (Isaka et al, 1992; Oosterlinck et al, 1993; Sekine et al, 1994; Solsona et al, 1999; Duque and Loughlin, 2000), and maintenance therapy does not reduce the risk further (Bouffioux et al, 1995; Tolley et al, 1996). Nevertheless, the level 1 data in support of single-dose MMC immediately after resection to reduce tumor recurrence are compelling (Table 81–3). Table 81–3 Successful Perioperative Administration of Intravesical Chemotherapy Modified from O’Donnell MA. Practical applications of intravesical chemotherapy and immunotherapy in high-risk patients with superficial bladder cancer. Urol Clin North Am 2005;32:121–31. A meta-analysis found that low-risk patients at a median follow-up of 3.4 years experience an approximate 39% drop in the odds of recurrence (i.e., from 48.4% to 36.7%). Patients with multiple tumors experience a 56% reduction in the odds of recurrence. MMC, epirubicin, and pirarubicin all significantly lessened the recurrence of both single and multiple tumors. Thiotepa did not show the same benefit, but data were limited and some studies used dilute strengths (Sylvester et al, 2004). The number needed to treat (NNT) to prevent one recurrence in the meta-analysis was 8.5, so some authors suggest that intravesical chemotherapy reduces overall cost of care by reducing the need for secondary resections. However, subsequent studies have shown that the tumors prevented are primarily smaller tumors that are often treated in the office or ambulatory surgery setting (Berrum-Svennung et al, 2008). In addition, the benefit appears limited to patients with low risk of recurrence (Gudjónsson et al, 2009), so the economic benefit regarding recurrences appears limited if treated in any manner other than inpatient care (Rao and Jones, 2009). Although local irritative symptoms are the most common complications of postoperative instillation, serious sequelae and rare deaths have occurred, especially in patients with perforation during resection (Oddens et al, 2004). Chemotherapy should be withheld in patients with extensive resection or when there is concern about perforation. Laser coagulation allows minimally invasive ablation of tumors up to 2.5 cm in size. The neodymium : yttrium-aluminum-garnet (Nd : YAG) laser has the best properties for use in bladder cancer. Lesions can be coagulated until nonviable through protein denaturation using a straight or 90-degree noncontact “free beam” laser using power output of up to 60 W. The most significant complication of laser therapy is forward scatter of laser energy to adjacent structures, resulting in perforation of a hollow, viscous organ such as overlying bowel. This is rare but most commonly occurs with the neodymium : YAG laser because of its deeper tissue penetration than with holmium : YAG and KTP lasers (Smith, 1986a, 1986b). Unless higher energy is necessary for a very large tumor, limiting energy to 35 W precludes exceeding 60° C on the outer bladder wall, minimizing the risk of perforation (Hofstetter et al, 1994). The most efficient delivery appears to be an end-fire noncontact fiber with a 5- to 15-degree angle of divergence, which allows variable penetration depth up to 5 mm (Smith and Landau, 1989; Holzbeierlein and Smith, 2000). Treatment should be under direct visualization and should discontinue as soon as protein denaturation is evident by the white appearance of the treated tissue. Persistence after this occurs risks extravesical injury. Laser therapy can be more expensive than resection due to the cost of laser fibers, but bleeding is negligible and there is no risk of obturator reflex. Small lesions can be treated easily using intravesical anesthesia. Because there is no tissue available for pathologic inspection, the optimal candidate for laser therapy is the patient with recurrent, low-grade lesions whose biology is already known. Additional information regarding tumor grade may be obtained with a cold cup biopsy if necessary. Some reports suggest lower recurrence rates using laser compared with TURBT, but this remains inconclusive (Smith et al, 1983; Malloy et al, 1984; Beisland and Seland, 1986; Smith, 1986a, 1986b; Beer et al, 1989). Holmium : yttrium-aluminum-garnet (YAG), argon, and potassium titanyl phosphate (KTP) lasers ablate tissues by cutting (vaporization) and thus have limited applicability due to lack of deep coagulation (Johnson et al, 1991; Benson, 1992; Holzbeierlein and Smith, 2000). The carbon dioxide laser is completely absorbed by fluid, so it is not appropriate for use in the treatment of bladder cancer (Benson, 1992). Many patients with small (<0.5 mL) low-grade recurrences can be managed safely in the office setting using diathermy or laser ablation under intravesical local anesthetic (Donat et al, 2004). Instillation of viscous or injectable 1% to 2% lidocaine through a catheter and a dwelling time of 15 to 30 minutes yield satisfactory mucosal analgesia. Pain with fulguration of 1- to 2-mm tumors is often acceptable with no analgesia. A tissue diagnosis and a negative cytology for the initial tumor occurrence are mandatory to determine whether the tumor is of high or low grade. Additionally, many small, low-grade tumors can be safely observed until they exhibit significant growth due to the minimal risk of progression (Soloway et al, 2003: Pruthi et al, 2008). These conservative approaches can obviate the need for anesthesia and use of hospital-based resources for selected small, low-grade recurrences. Endoscopically, urologists can suspect malignancy only on the basis of the presence of visible changes such as tumors or “red spots.” As noted, random biopsy of normal-appearing areas sometimes detects unsuspected malignancy, usually CIS. Moreover, a multicenter study found that 37% of the biopsies performed on the basis of suspicious endoscopic findings resulted in false-negative biopsy, emphasizing the subjectivity and attendant false-positive and false-negative rates of cystoscopy (Riedl et al, 2001; Sarosdy et al, 2002). The imperfect sensitivity of cystoscopy potentially explains the high rate of cancer recurrence soon after complete removal of all visible tumors (tumor cell implantation also contributing, as described earlier). It is likely that cancer was already present but not visible at the time of resection and simply became visible in follow-up when it became morphologically abnormal enough to differentiate from adjacent normal urothelium. Photoactive porphyrins accumulate preferentially in neoplastic tissue. Under blue light they emit red fluorescence, which can help in the diagnosis of indiscernible malignant lesions. Hematoporphyrin derivatives must be administered systemically and can cause lengthy, residual cutaneous photosensitization. Intravesical application of 5-aminolevulinic acid (5-ALA), a precursor of photoactive porphyrin, avoids residual systemic photosensitization and has improved the detection of bladder tumors. Modifications of 5-ALA may allow deeper tissue penetration and improved accumulation in neoplastic cells (Lange et al, 1999). A more lipophilic ester, hexaminolevulinate (HAL), is the most studied agent. When using this technology, both small papillary tumors and almost one-third more cases of CIS overlooked by cystoscopy are identified (Jichlinski et al, 2003; Schmidbauer et al, 2004; Fradet et al, 2007). Of all tumors, 96% were detected with HAL imaging compared with 77% using standard cystoscopy. Detection was improved for dysplasia (93% vs. 48%), CIS (95% vs. 68%), and papillary tumors (96% vs. 85%) (Jocham et al, 2005). The clinical impact of improved tumor detection seems intuitive, and prospective evidence shows that this decreases recurrence rates in patients who undergo HAL fluorescence cystoscopy compared with controls (Filbeck et al, 2003; Denzinger et al, 2007). Intravesical HAL for use with fluorescence cystoscopy received approval for use in Europe in 2004. A multinational study to determine the actual impact on recurrence rates before approval in North America showed similar findings (unpublished data) (Figs. 81-6 and 81-7). Figure 81–6 White light microscopy reveals normal appearing mucosa. (Courtesy of H. Barton Grossman, MD.) (Courtesy of H. Barton Grossman, MD.) Key Points: Endoscopic Surgical Management Intravesical immunotherapy results in a massive local immune response characterized by induced expression of cytokines in the urine and bladder wall and by an influx of granulocytes, mononuclear, and dendritic cells (Shen et al, 2008). The mechanism of action has been intensively investigated. The initial step appears to be direct binding to fibronectin within the bladder wall, subsequently leading to direct stimulation of cell-based immunologic response and an antiangiogenic state. Numerous cytokines involved in the initiation or maintenance of inflammatory processes including tumor necrosis factor (TNF)-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, and interleukin (IL)-1, IL-2, IL-5, IL-6, IL-8, IL-10, IL-12, and IL-18 have been detected in the urine of patients treated with intravesical BCG and other immunostimulatory agents. The observed pattern of cytokine induction with preferential upregulation of interferon-γ, IL-2, and IL-12 reflects induction of a T-helper type-1 (Th1) response. This immunologic response activates cell-mediated cytotoxic mechanisms that are believed to underlie the efficacy of BCG and other agents in the prevention of recurrence and progression (Bohle and Brandau, 2003). In addition, BCG may concomitantly stimulate IL-10, resulting in the suppressive T-helper type-2 (Th2) response. Increasing the predominance of the Th1 response versus the Th2 response is an area of continued research (Lou, 2003). Overall, response to intravesical immunotherapy may be limited if a patient has an immunosuppressive disease or by advanced age (Joudi, 2006a, 2006b). BCG is an attenuated mycobacterium developed as a vaccine for tuberculosis that has demonstrated antitumor activity in several different cancers including UC (Morales et al, 1976). The original regimen described by Morales included a percutaneous dose, which was discontinued after success using a similar intravesical regimen by Brosman (1982). BCG is stored in refrigeration and reconstituted from a lyophilized powder. Connaught, Tice, Armand Frappier, Pasteur, Tokyo, and RIVM strains all arise from a common original strain developed at the Pasteur Institute. The vaccine is reconstituted with 50 mL of saline and should be administered through a urethral catheter under gravity drainage soon thereafter to avoid aggregation (Ratliff et al, 1994). Treatments are generally begun 2 to 4 weeks after tumor resection, allowing time for re-epithelialization, which minimizes the potential for intravasation of live bacteria (Lamm et al, 1992b). For the same reason, a urinalysis is usually performed immediately before instillation to further ensure a diminished probability of systemic uptake of BCG. In the event of a traumatic catheterization, the treatment should be delayed for several days to 1 week, depending on the extent of injury. After instillation, the patient should retain the solution for at least 2 hours. Some clinicians have advocated the patient turn from side to side to bathe the entire urothelium, but there is no scientific support for this practice. Fluid, diuretic, and caffeine restriction before instillation is essential to limit dilution of the agent with urine and to facilitate retention of the agent for 2 hours (Lamm et al, 2000a). Patients are instructed to clean the toilet with bleach, although there is no demonstrable risk of close contact infection. American urologists use BCG by a 2 : 1 margin compared with intravesical adjuvant or maintenance chemotherapy (e.g., doxorubicin hydrochloride [Adriamycin], gemcitabine, thiotepa), whereas European urologists favor chemotherapy. BCG is approved for this indication by the U.S. Food and Drug Administration (FDA). Before the adoption of BCG intravesical therapy, CIS reportedly progressed at an average rate of 7% per year (Zinke, 1985).The initial tumor-free response rate is as high as 84% (Brosman, 1982; DeJager et al, 1991; Hudson and Herr, 1995; Lamm et al, 2000a, 2000b, 2000c). Approximately 50% of patients experience a durable response for a median period of 4 years. Over a 10-year period, approximately 30% of patients remain free of tumor progression or recurrence, so close follow-up is mandatory. The majority of these occur within the first 5 years (Herr et al, 1992). Herr and coworkers (1989) reported progression in 19% of initial responders at 5 years but found the rate to be 95% in nonresponders—findings confirmed by other investigators (Coplen et al, 1990; Harland et al, 1992). The American Urological Association (AUA) Guidelines Panel supported BCG as the preferred initial treatment option for CIS (Hall et al, 2007). BCG has gained a pre-eminent role in North America on the basis of higher efficacy reports compared with intravesical chemotherapy, despite greater morbidity than chemotherapy (O’Donnell, 2007). In more than 600 patients, there was a 68% complete response rate to BCG and a 49% complete response rate to chemotherapy. In responders, 68% of patients treated with BCG remained free of disease as compared with 47% of patients receiving chemotherapy, on the basis of a median follow-up of 3.75 years. The overall disease-free rates were 51% and 27%, respectively (Sylvester et al, 2005). Intravesical BCG can effectively treat residual papillary lesions but should not be used as a substitute for surgical resection. Investigators have demonstrated a nearly 60% response by residual tumor with intravesical BCG alone (Brosman, 1982; Schellhammer et al, 1986; Coplen et al, 1990). Carcinoma of the mucosa or the superficial ducts of the prostate can be adequately treated by BCG with a 50% tumor-free rate. A limited TURP can be effective in decreasing tumor burden and facilitating exposure of the prostate surface to BCG administration (Bretton et al, 1990; Schellhammer et al, 1995). Early single-center studies demonstrated an advantage in decreased tumor recurrence of approximately 30% when a 6-week course of BCG was administered after recovery from TURBT (Brosman, 1982; Morales et al, 1992). In several larger series, tumor recurrence after TURBT was reduced by 20% to 65%, for an average of approximately 40% (Pagano et al, 1991a, 1991b; Herr et al, 1992; Melekos et al, 1993; Krege et al, 1996). Han and Pan published a meta-analysis in 2006 evaluating 2000 patients with Ta, T1, and/or CIS disease. Patients receiving maintenance BCG had a statistically decreased rate of recurrence compared with those receiving induction therapy alone (Han and Pan, 2006). The efficacy of BCG after TURBT for high-risk papillary disease has been demonstrated in several series of T1 lesions, with recurrence rates of 16% to 40% and progression rates of 4.4% to 40%, a substantial improvement compared with TUR alone (Cookson and Sarosdy, 1992; Pansadoro et al, 1995; Herr, 1997; Jimenez-Cruz et al, 1997; Gohji et al, 1999; Hurle et al, 1999a, 1999b). Tumor multiplicity and associated CIS were associated with increased risk of progression. Substaging lesions on the basis of the presence or absence of muscularis mucosae invasion in a series of 49 patients did not improve prediction of recurrence (69% vs. 65%) or progression (22% vs. 29%) after BCG therapy (Kondylis et al, 2000). Although reports of the impact of BCG on tumor recurrence are compelling, the greater need is the potential for impact on progression. In 403 patients with CIS, BCG reduced the risk of progression by 35% compared with intravesical chemotherapy (Sylvester, 2002). In a randomized trial of 86 patients with high-risk superficial disease, Herr and colleagues (1988) demonstrated a greater delay in interval progression for BCG patients versus TUR controls. Additionally, the cystectomy rate was significantly decreased for CIS patients treated with BCG (11% vs. 55% for controls), as was the time to cystectomy. However, only 27% of patients were alive with an intact functioning bladder after follow-up over 10 to 15 years, so this apparent advantage is temporary in many cases (Cookson et al, 1997). Available data suggest that BCG can delay progression of high-risk bladder cancer, yet the long-term survival advantage is hypothetical. Nevertheless, two meta-analyses have concluded that BCG reduces the risk of progression. Progression at 2.5 years’ median follow-up was reduced by 27% (9.8% for BCG vs. 13.8% for non-BCG) in one (Sylvester, 2002) and by 23% (7.7% for BCG vs. 9.4% for MMC) at 26-month median follow-up in another analysis (Bohle and Bock, 2004). In both cases the superior results with BCG were only seen in trials using BCG maintenance therapy. In contrast, no chemotherapy trials have achieved a significant reduction in progression (Grossman et al, 2008). Nuclear P53 overexpression before BCG therapy has not been shown to predict response to therapy, but post-therapy P53 overexpression is an independent marker of disease progression (Lacome et al, 1996; Lebret et al, 1998). The AUA Guidelines panel concluded that BCG appeared likely to reduce progression (Hall et al, 2007). The optimal treatment schedule and dose for BCG have not been established. Morales wrote in the reply to an editorial comment to his landmark article (see box), “This regimen is arbitrary, and may be modified in the future as additional data become available” (Morales et al, 1976). In reality, several studies suggest that a 6-week induction course alone is insufficient to obtain an optimal response in many patients and that maintenance therapy is requisite (Lamm et al, 2000a, 2000b, 2000c; Palou et al, 2001). The average additional response to a second induction course is 25% in those patients treated for prophylaxis and 30% in CIS patients (Haaff et al, 1986a, 1986b; Kavoussi et al, 1988; Bretton et al, 1990; Coplen et al, 1990; Sylvester, 2002; Bohle and Bock, 2004). However, additional courses of BCG to treat refractory patients after a second 6-week course are accompanied by a significant risk of tumor progression in 20% to 50% of patients (Nadler et al, 1994). Catalona and colleagues (1987) reported roughly a 7% actuarial risk of progression with every additional course of BCG therapy. Response to BCG at 6 months can be used as a predictor of prognosis, with the number of patients developing progressive disease being significantly higher among nonresponders (Orsola et al, 1998). The Southwest Oncology Group (SWOG) reported the most significant impact of maintenance therapy. Patients received a 6-week induction course followed by three weekly instillations at 3 and 6 months and every 6 months thereafter for 3 years. Estimated median recurrence-free survival was 76.8 months in the maintenance arm and 35.7 months in the control arm (P = .0001). Average recurrence-free survival was 111.5 months in the control arm and not able to be estimated in the maintenance arm (P = .04). Overall 5-year survival was 78% in the control arm and 83% in the maintenance arm. No toxicities above grade 3 were observed, yet only 16% of patients tolerated the full dose-schedule regimen. Two thirds of the patients who stopped BCG due to side effects did so in the first 6 months, suggesting that the side effects do not increase appreciably with additional time on therapy. An interpretation that the intended full course of maintenance therapy cannot be accomplished in most patients due to side effects is misleading. Due to the fact that the treatment group fared better despite most patients failing to complete the full course of therapy, the maximum benefit may have been achieved earlier. Shorter maintenance schedules and reduced dosages may accomplish the same results with less toxicity (Lamm et al, 2000b). Although some older studies failed to identify a benefit to maintenance therapy (Badalament et al, 1987), most authorities believe that at least 1 year of maintenance therapy is appropriate. Clearly for high-grade T1 lesions or CIS, maintenance therapy has proven superior in multiple studies (Palou et al, 2001). The determination of whether the optimal treatment schedule should be as described in the SWOG study or monthly remains unclear, and optimal duration of a monthly maintenance schedule, if chosen, is unknown (Lamm et al, 2000a, 2000b, 2000c; O’Donnell, 2005). The authors routinely administer the first of three maintenance doses immediately after the scheduled cystoscopy confirms absence of recurrence, with the two following doses weekly thereafter. Several investigators have evaluated the potential for BCG dose reduction (Morales et al, 1992; Melekos et al, 1993; Martinez-Pineiro et al, 1995; Pagano et al, 1995). In general, a decrease in toxicity with no statistical difference in efficacy has been noted in small series (Pagano et al, 1991b; Mack and Frick, 1995; Hurle et al, 1996), although multifocal and high-grade tumors may respond better to full dosing (Martinez-Pineiro et al, 2002). Some studies have shown an upregulation of the Th1 response with a lower-dose BCG. Lengthening of the instillation interval may decrease side effects without loss of efficacy (Bassi et al, 2000). European studies, where BCG inoculation for tuberculosis is more common than in North America, suggest the dose may be safely reduced by half (Martinez-Pineiro et al, 2002). The difference in response to doing so in immunologically naive North Americans is unknown, but Morales and colleagues (1992) found a significant decrease in response rates (67% vs. 37%), especially for patients with CIS in combination with papillary tumors treated with the reduced dose. Antibiotic therapy may have a beneficial effect in treating or preventing systemic side effects of BCG therapy, yet it may also inhibit the effectiveness of BCG therapy if it is given routinely for urinary tract prophylaxis during a course of BCG therapy (Durek et al, 1999a, 1999b). Quinolones in particular may affect the viability of BCG and should be avoided if possible during the course of BCG treatments (Durek et al, 1999b). In contrast, in vitro data suggest that quinolone antibiotic therapy may augment intravesical chemotherapy with agents such as doxorubicin because both agents affect topoisomerase II inhibitors (Kamat et al, 1999) (Tables 81-4 and 81-5). Table 81–4 Contraindications to Bacillus Calmette-Guérin (BCG) Therapy
Staging
Pathology: Staging and Grading
Pathologic Staging
Tumor Biology
Pathologic Characteristics by Stage and Implications for Clinical Staging Errors
STUDY
INSTITUTION
RISK (%) OF UNDERSTAGING
Stein et al, 2001
Southern California
39
Dutta et al, 2001
Vanderbilt University
40
Bianco et al, 2004
Wayne State University
27
Bayraktar et al, 2004
Vakif Gureba Hospital
50
Aksaray-Istanbul, Turkey
Huguet et al, 2005
Servicio de Urologia,
27
Fundacion Puigvert, Barcelona
Ficarra et al, 2005
University of Verona, Italy
43
Endoscopic Surgical Management
Complications of Transurethral Resection of Bladder Tumor
Repeat Transurethral Resection of Bladder Tumor
Role of “Random” or Additional Biopsies
Perioperative Intravesical Therapy to Prevent Tumor Implantation
Laser Therapy
Office-Based Endoscopic Management
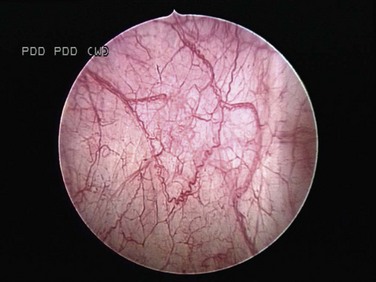
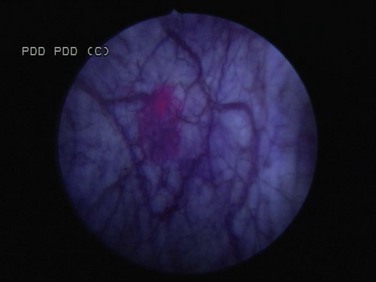
Fluorescence Cystoscopy
Immunotherapy
Bacillus Calmette-Guérin
BCG Treatment of Carcinoma in Situ
BCG Treatment of Residual Tumor
BCG Prophylaxis to Prevent Recurrence
Impact of BCG on Progression
Determining Optimum BCG Treatment Schedule
Absolute Contraindications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

Non–Muscle-Invasive Bladder Cancer (Ta, T1, and CIS)
• TURBT is performed both to remove all visible tumors and to provide specimens for pathologic examination to determine stage and grade.
Immediately after transurethral resection on the basis of the risk of intravasation and septic death