Introduction
Over the last decade, the major developments in renal-transplant-surgery techniques have been related to laparoscopic live-donor nephrectomy. However, in more recent years there have also been advances in surgical techniques in the recipient, particularly with regard to minimally invasive and robotic-assisted surgery.
This chapter discusses the various techniques for live-donor nephrectomy in detail, including the use of robotic-assisted surgery. Recent trends in the perioperative management of live donors are also examined. Finally, new techniques in recipient surgery are explored, including minimally invasive approaches, approaches to anastomosing multiple arteries, and the use of suture-free vascular anastomosis devices.
Live-donor nephrectomy: surgical techniques
Over recent years, living-donor renal transplantation has increasingly been used to offset the shortage of cadaveric kidneys. The surgical technique in the donor has evolved over this time from open nephrectomy via a flank incision with rib resection, through short-muscle-splitting incision without rib resection, to laparoscopic minimally invasive nephrectomy.
In the UK there has been a rapid increase in the number of transplant units performing laparoscopic donor nephrectomy. A recent survey conducted in Leicester has shown that over 80% of units now offer laparoscopic donor nephrectomy (unpublished data), as compared to 21% in 2002 [1]. This rise is based on good evidence of its benefit over traditional open surgery from randomized clinic trials and meta-analyses.
There are a number of different minimally invasive approaches, namely total laparoscopic donor nephrectomy (LDN), hand-assisted laparoscopic donor nephrectomy (HALDN), retroperitoneoscopic donor nephrectomy (RDN) and hand-assisted retroperitoneoscopic donor nephrectomy (HARDN).
Total laparoscopic donor nephrectomy
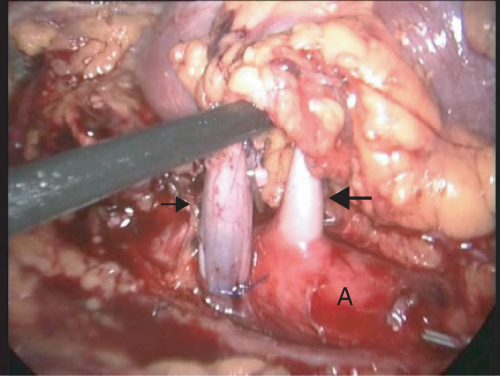
This procedure is performed with the donor in the lateral decubitus position, with the kidney to be removed uppermost. A 10–12 mm port is introduced either by direct vision or following Verress needle insufflation. Capnoperitoneum is established at 12–15 cm H2O pressure and a 30° endoscope is introduced. Three to four additional 10–12 mm or 5 mm ports are inserted. The procedure begins by mobilizing and medially displacing the colon overlying the kidney; this dissection can be facilitated by the use of an ultrasonic or other electrosurgical dissector. The ureter and gonadal vein are then identified at the pelvic brim. The gonadal vein is followed superiorly to identify the renal vein. This is dissected free and its branches are controlled and divided. Our practice is to suture ligate the venous branches, utilizing laparoscopic knot-tying. This avoids metal clips adjacent to the renal vasculature, which might interfere with stapling of the vessels. The renal artery is identified posterior to the renal vein. On the left, this is dissected down to its origin at the aorta (see Figure 2.1) and on the right to the lateral border of the inferior vena cava or just beyond. If there are concerns regarding the length of the right renal artery, retrocaval dissection can be performed to allow division of the artery closer to its origin from the aorta. This is not for the fainthearted, however, as there is a risk of damage to posterior lumbar veins draining into the posterior cava. The resultant bleeding can be difficult or impossible to control laparoscopically, necessitating conversion to an open procedure. Following dissection of the renal vessels, the inferior, lateral, and superior attachments of the kidney are divided until the kidney is isolated on its vessels and ureter.
Figure 2.1 Intraoperative photograph during left LDN showing dissection of the renal vein (small arrow) and of the renal artery (large arrow) down to the aorta (A)
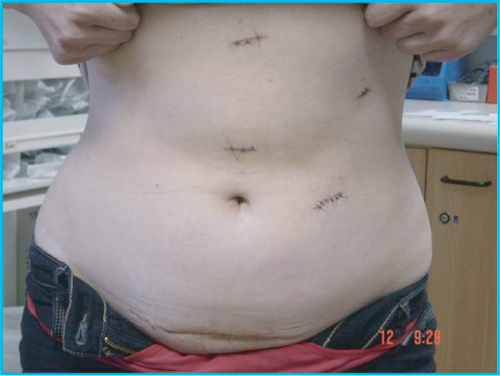
A 6 cm Pfannenstiel incision is then made, followed by a small incision in the peritoneum, and an endoscopic retrieval bag is introduced into the abdominal cavity. A purse-string suture is placed around the peritoneum to maintain pneumoperitoneum. The ureter is first clipped and divided, and the renal vessels are then clipped or stapled. The kidney is placed in the bag and retrieved through the Pfannenstiel incision; it is then removed to the back table and flushed with preservative solution. Repeat laparoscopy is performed to ensure hemostasis prior to withdrawal of the ports and closure of the incisions. The resultant scars are shown in Figure 2.2.
The evidence
Alongside many nonrandomized studies, six randomized controlled trials [2–7] and two meta-analyses [8–9] have compared LDN with open techniques. Overall, these high-level studies have concluded that LDN results in significantly less analgesic requirements, shorter hospital stay (by 1.6 days, p < 0.001) and return to work (by 2.4 weeks, p < 0.001), and less chronic wound pain than open techniques, with similar complication rates [9]. However, this is at the expense of significantly longer operative times and, in some studies, increased first warm-ischemic time. Nevertheless, this does not appear to impact on graft outcome in the transplant recipients.
Hand-assisted laparoscopic donor nephrectomy
HALDN is performed in a similar fashion to LDN, but differs in that a hand port is placed at the extraction site. This can be done either at the beginning of the procedure or only for control of the vessels and extraction of the kidney. The hand port allows better tactile feedback, digital dissection, easier and quicker control of bleeding, and potentially quicker kidney extraction. Many surgeons feel HALDN improves the safety of LDN of donors, particularly when training or early in one’s experience of LDN. Various incisions for the hand port have been described, including periumbilical, Pfannenstiel, and midline infraumbilical.
The evidence
The majority of (nonrandomized) studies comparing LDN with HALDN have concluded that HALDN results in shorter operating times, hospital stay, and first warm-ischemic time, with less blood loss [10–16]. However, the only randomized study comparing LDN with HALDN found that operating times were significantly longer in the HALDN group, with no difference in blood loss, warm-ischemic time, or length of hospital stay; this trial included only 40 patients, so it is difficult to draw firm conclusions [17]. There is also a study comparing right and left approaches after HALDN, showing no significant differences other than a shorter operating time for right kidneys [18].
Retroperitoneoscopic donor nephrectomy
This is performed with the patient in the lateral decubitus position. Following insertion of the initial port, the operating space is created by either digital dissection or using a balloon dilator. This space must extend to above the upper pole of the kidney and medially to the midline. The retroperitoneal space is maintained using CO2 at a maximum pressure of 12 cm H2O and a further two or three trocars are inserted. The retroperitoneal approach obviates any mobilization of the colon or spleen. The anteromedial portion of Gerota’s fascia is opened and the upper pole of the kidney is dissected free. Attention is then turned to the renal vasculature, with the renal vein dissected free and its branches secured and divided. The artery is dissected down to the aorta on the left, or to the level of the inferior vena cava on the right. The ureter is then dissected down to the iliac vessels, and the lateral attachments of the kidney are divided. The ureter and vessels are divided and the kidney is removed via a preformed Pfannenstiel or lower midline incision.
The limited retroperitoneal space makes RDN potentially more technically challenging than LDN. This may be resolved somewhat by using a flexible laparoscope to improve visualization. In addition, tears in the peritoneum during dissection may result in escape of gas into the peritoneal cavity, reducing the retroperitoneal working space further. This can be remedied by placing a Verress needle into the peritoneal cavity to vent the escaped gas.
Nevertheless, the technical advantage of RDN is improved access to the retrorenal space, which particularly helps with managing posterior lumbar veins draining into the left renal vein and improves access to the retrocaval renal artery on the right. In addition, the peritoneal cavity is not breached, reducing the risk of bowel injury and intraabdominal adhesions.
The evidence
There have been no randomized controlled trials comparing RDN with either LDN or open techniques. The available case-control studies comparing RDN with open techniques have reported differing results in terms of operative time and warm-ischemic time, although RDN does appear to be associated with less postoperative pain [19–20]. The limited studies comparing RDN with LDN suggest equivalence between the two techniques in terms of postoperative pain, operative time, and warm-ischemic time [21–22].
As with LDN, hand-assisted RDN (HARDN) can also be performed, with the hand port placed at the Pfannenstiel extraction site. Again, HARDN has not been subjected to vigorous evaluation by randomized controlled trials, although recruitment to a multicenter study is currently taking place [23]. However, nonrandomized comparisons do appear to suggest that HARDN is significantly quicker than LDN [24–25].
Robotic-assisted laparoscopic donor nephrectomy
The use of robotic assistance has a number of advantages over conventional laparoscopic surgery, mainly related to visibility and dexterity. The instruments of the robotic devices have far greater degrees of movement and articulation than standard laparoscopic instruments and any hand tremor is filtered out by software. This allows more precise, delicate maneuvers and dissection. In addition, it enables restoration of the three-dimensional vision lost in standard laparoscopic surgery.
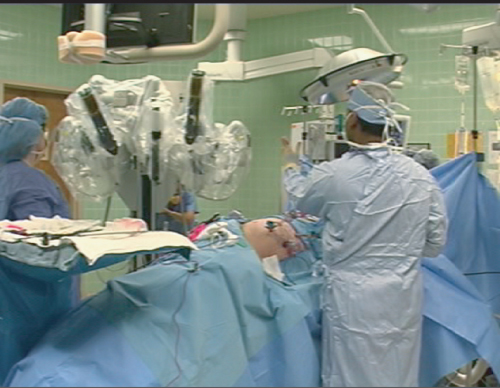
The technique for RALDN is as follows. The donor is placed in the lateral decubitus position. Four 10–12 mm laparoscopic ports are used: two for the robotic instruments, one for the laparoscope, and one for the assistant. Once the robotic instruments are docked as shown in Figure 2.3, the surgeon is seated at the remote console. The assistant remains at the operating table and is responsible for instrument exchanges, suction-irrigation, clip application, and insertion or extraction of the endoscopic retrieval bag. The dissection and extraction of the kidney proceeds as described for LDN.
The evidence
The first report of RALDN was published in 2002 from the University of Illinois at Chicago, describing 10 successful cases [26]. Since then, a number of other centers have reported their experience. Hubert et al. described RALDN in 38 donors, with a mean operating time of 181 minutes, no conversions, and no intraoperative complications [27]. The University of Illinois at Chicago has reported its further experience with 209 left RALDNs [28]. These included 61 patients with multiple renal vessels requiring back-table reconstruction. Mean operating time was 152 minutes, delayed graft function was 0%, open conversion for bleeding was necessary in four patients (2%), and mean length of stay for the donor was 2 days.
However, there are no randomized controlled trials of RALDN, and currently there is no clear evidence of any clinical benefit from robotic-assisted procurement.
The greatest barrier to RALDN is currently the cost of purchasing and maintaining the robotic equipment. The only commercial robotic-surgery system currently available is the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) (Figure 2.3), which has a cost of over US$1.25 million. Unless this figure drops dramatically, this technology is going to be unavailable to the vast majority of transplant centers, particularly without evidence of resounding clinical or cost benefits over conventional laparoscopic donor nephrectomy.
Single-port donor nephrectomy
Laparoendoscopic single-site (LESS) surgery is a recent development in minimally invasive surgery. This technique employs a single-access multichannel port such as the TriPort (Olympus Keymed, Southend-on-Sea, UK) or SILS port (Covidien, Dublin, Ireland). These ports are inserted through a 2–3 cm Pfannenstiel or vertical intraumbilical incision and allow simultaneous passage of a laparoscope and two instruments. The use of curved and articulating laparoscopic instruments prevents clashing of instruments and restores some of the triangulation lost due to the close insertion points.
Intraabdominally, the LESS LDN mirrors the conventional LDN. The kidney is extracted in an endobag through an extension of the LESS incision.
The evidence
To date, at least seven centers have reported their experience of LESS surgery for donor nephrectomy. These have confirmed the feasibility of the technique. Of the two studies comparing LESS with conventional LDN, one reports no difference between the groups in terms of operative time, warm-ischemic time, analgesia requirements, and pain scores, while the other shows a significantly quicker return to work and full physical recovery in the LESS group [29–30]. A recent randomized controlled trial comparing LESS with conventional LDN in 50 patients found LESS to result in significantly longer warm-ischemic times (5 versus 7 minutes, p < 0.0001) but slightly shorter hospital stay (4.6 versus 3.9 days, p = 0.003). The estimated glomerular filtration rate at 1 year was comparable between the groups [31].
Transvaginal extraction of the kidney
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree