Introduction
Pancreas transplantation has become a more applicable option for treating insulin-dependent diabetes mellitus over the last 3 decades [1].
Type-1 diabetes mellitus has two treatments: (a) exogenous insulin administration or (b) β-cell replacement by pancreas or islet transplantation. The former is burdensome to the patient and gives imperfect glycemic control, predisposing to secondary complications of the eyes, nerves, kidneys, and other systems. The latter, when successful, establishes a constant euglycemic state but requires major surgery—at least for the pancreas transplant—and immunosuppression to prevent rejection, predisposing to complications as well, often compounded by those that are preexisting from diabetes.
Because of the established lack of sustained success with islet transplantation [2], solid-organ pancreas transplantation remains the gold standard for β-cell replacement. With refinement in surgical techniques, the availability of better immunosuppression, and lessons learned from previous experience, the results of pancreas transplantation have improved significantly [1,3–5]. This improvement has brought a paradigm shift in the current approach towards a patient with diabetes mellitus, in that the main aspects considered are the overall surgical/anesthetic candidacy and the benefit in trading off the need for insulin administration to that of immunosuppression.
Diabetes mellitus
According to the World Health Organization (WHO), the age-standardized worldwide adult diabetes prevalence in 2008 was 9.8% (range 8.6–11.2) in men and 9.2% (8.0–10.5) in women, which has gone up from 8.3% (6.5–10.4) and 7.5% (5.8–9.6) in 1980. The number of people with diabetes increased from 153 (127–182) million in 1980 to 347 (314–382) million in 2008 [6]. This number is expected to touch 438 million by 2030. The current prevalence ranges from 10.2% in the Western Pacific to 3.8% in the African region, and diabetes is a leading cause of premature illness and death worldwide.
According to the American Diabetes Association [7], the prevalence of diabetes in Americans aged 60 or more is about 18.3%. Diabetic nephropathy accounts for 40% of newly diagnosed cases of end-stage renal disease in the USA. It is seen in 20–30% cases of type-1 or 2 diabetics, but a greater proportion of those with type 1 deteriorate to require dialysis. However, given the significantly larger number of type-2 diabetics in comparison to type-1, the former account for nearly half of the diabetics requiring dialysis. And among dialysis patients, those with diabetes have higher annual costs and mortality [8].
Diabetes management
Insulin versus pancreas transplantation
The requirement for insulin administration affects the quality of life of an individual. Not only is it a burden, but there is often suboptimal control of blood sugars, which can lead to the development of microvascular complications affecting the kidneys, eyes, and nerves, to name but a few. Studies have shown that intensive insulin therapy does improve, but rarely normalizes, glycosylated-hemoglobin levels and helps in the reduction of diabetic microvascular complications [4,9,10]. Perfect glycemic control is extremely difficult to achieve even with the most rigorous and meticulous insulin delivery options, and even if achieved, rates of secondary complications remain high [11,12]. On the other hand, it can be achieved through solid-organ pancreas transplantation, with normalization of glycosylated hemoglobin levels [13,14]. Pancreas transplantation can restore the production of endogenous insulin, and also help in the counterregulation of glucose metabolism. There is normalization of glucose metabolism at cellular and systemic level, and normalization of glucose homeostasis, translating into avoidance of hyperglycemia and hypoglycemia episodes [15,16]. With the patient rendered insulin-free through a successful pancreas transplant, there is also an improvement in quality of life, a reduction as well as reversal of some microvascular complications, and an elimination of the cumbersome process of daily insulin injections and frequent glucose monitoring. In the long term, it stabilizes retinopathy, improves neuropathy, causes regression of nephropathy by improving glomerular architecture, reverses interstitial expansion, resorpts atrophic tubules, and improves macrovascular disease, cardiac function, and endothelial function [17–26]. These improvements occur in addition to the alleviation of anxiety relating to frequent administration of insulin. However, there is no improvement in advanced vascular disease and retinopathy [27]. Therefore, the advantages of transplanting a pancreas are greater if it is done before the onset of severe complications.
Pancreas transplantation versus islet-cell transplantation
Islet-cell transplantation, another type of β-cell replacement, is usually performed using deceased-donor pancreata. Current criteria in the USA preferentially allocate donors over age 50 and with body mass index (BMI) >30 to islet transplants. The process entails extraction of islet cells from the pancreas by enzymatic digestion of the parenchyma, and subsequent purification to obtain the islet-cell preparation. The islet cells are then injected into the portal vein of the recipient, usually by radiological or minimally invasive surgical techniques.
Interest in islet transplantation was revived in the late 1990s after the “Edmonton Protocol,” using a steroid-free nondiabetogenic immunosuppression protocol [28] that showed high insulin-independence rates at 1 year post-transplant. Similar results from single-donor islet-cell transplants have been reported from the University of Minnesota, utilizing a similar regimen, but these included donors with a high BMI whose islets were transplanted to recipients with a lower BMI, thereby transplanting a similar net islet number per unit weight to that of the Alberta series [29].Currently, the success rates of insulin independence, although promising in the short term, tend to taper in the long term, with graft function—even after multiple-donor islet-cell transplants—about 10% at 5 years post-transplantation [30]. The suboptimal success rates have been attributed to a variety of causes [31], but the results are continuing to improve [2,32,33]. A recent Collaborative Islet Transplant Registry (CITR) report showed significantly improved islet-graft survival rates [2]. This study found that among 325 adult recipients of 649 islet infusions derived from 712 donors, at 3 years post-first infusion, 23% of islet-alone recipients were insulin-independent, 29% were insulin-dependent (but with detectable C-peptide), and 26% lost function. The remaining 22% had missing data. About 70% achieved insulin independence at least once, of whom 71% maintained status quo 1 year later and 52% at 2 years. However, the success rates were favored by higher numbers of infusions and of total islet equivalents infused, lower pretransplant glycosylated hemoglobin levels, processing centers related to the transplant center, and larger islet size. Use of protocols with daclizumab or etanercept during induction lowered the rates of function loss and achieved higher rates of insulin-independence.
Until islet transplants can consistently succeed from a single donor, regardless of recipient size or insulin requirements, an integrated approach is likely; large donors will be used for islet transplants to recipients with low insulin needs, and the remaining donors (the majority) for pancreas transplants to recipients with average or high insulin requirements. This strategy will maximize the number of recipients who receive allogeneic β-cells and eliminate surgical complications for at least a subset of patients.
Pancreas transplantation: a routine technique?
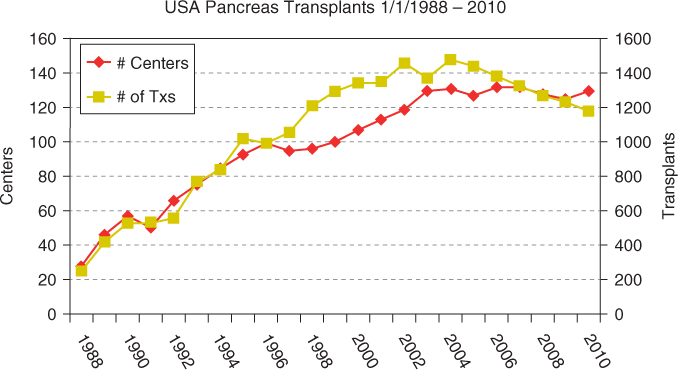
Around 35 000 pancreas transplants have been performed worldwide, of which 24 000 were performed in the USA (Figures 5.1 and 5.2). Currently, about 1200 pancreas transplants are performed annually in the USA, and about 800 in the rest of the world. Of these, 75% are simultaneous pancreas–kidney (SPK) transplants, 18% are pancreas after kidney (PAK) transplants, and 7% are pancreas transplants alone (PTAs). Despite an initial increase in numbers, pancreas transplants have declined since 2004, with the largest decrease seen in the PAK category (by 50%), and a decline in SPKs of 7% from 2004 to 2010. An analysis of US pancreas transplantation over different eras showed that the mean recipient age at transplantation increased from 25–35 years in 1988 to about 40 years in 2010. There has also been an increase in pancreas implants into type-2 diabetics (currently 8% for SPK, 5% for PAK, and 1% for PTA). However, donor selection has become more stringent, with an increasing number of transplants using younger donors and trauma victims, and with shorter preservation time (mean 10–12 hours currently). There has been an increase in pancreas retransplants (currently 2% for SPK, 22% for PAK, and 18% for PTA), African-American recipients (currently 18% for SPK and 6% for PAK and PTA). Enteric drainage is currently the predominant duct-drainage technique (90% for SPK and PAK and 84% for PTA), and systemic venous drainage is the predominant venous drainage used (82% for SPK and PAK and 90% for PTA). Immunosuppressive protocols commonly use antibody induction with tacrolimus/mycophenolate mofetil as major maintenance therapy. Steroid avoidance has also increased in all three categories of pancreas transplant (30% for SPK and 48% for PAK and PTA).
Figure 5.1 Pancreas transplants performed in the USA and worldwide. Reproduced with permission from Angelika Gruessner, International Registry of Pancreas Transplantation
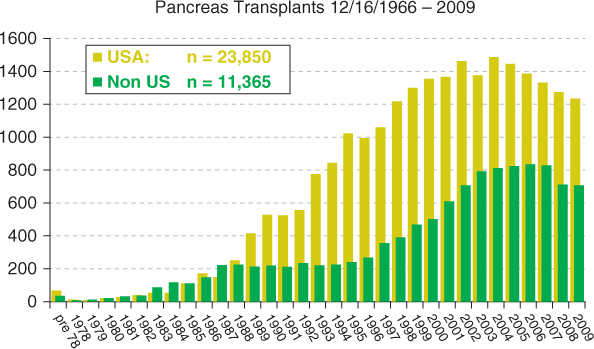
Figure 5.2 Number of transplant (Tx) centers and number of pancreas transplants in the USA. Reproduced with permission from Angelika Gruessner, International Registry of Pancreas Transplantation
Indications and contraindications for pancreas transplantation
The indication for pancreas transplantation differs for diabetic patients with normal renal function versus those with renal failure. For those with normal renal function, the foremost indication is hypoglycemia unawareness and labile diabetes with frequent insulin reactions. In diabetics whose autonomic nerves have been affected by longstanding hyperglycemia [34], episodes of hypoglycemia unawareness may occur, where the usual neural and cardiac warning signs of impending low sugars, such as restlessness, anxiety, and sense of impending death, are masked and they are unable to take remedial measures. This can potentially pose a threat to life (self or others) or cause irreversible brain damage. In those who have labile diabetes, the risks of a surgical procedure and consequences of immunosuppression may be preferable to the burden of a lifetime of diabetes [12,35]. Even among those without the above mentioned symptoms, many would prefer to have a pancreas transplant instead of carrying a lifetime burden of managing diabetes with insulin. In these candidates, however, it is unclear whether the long-term risks of diabetes are overcome by the risks of immunosuppression.
In contrast, the uremic diabetic is frequently considered for pancreas transplantation, in addition to kidney [36]. The risks of immunosuppression are already assumed because of the need for a kidney transplant, so a simultaneous or sequential pancreas transplant does not pose significant additional risks, other than surgical ones. Traditionally, uremic individuals with type-1 diabetes are offered a pancreas along with a kidney [36]. Recent literature on transplantation of the pancreas in type-2 diabetics is also promising [37–39]. It has been noted that survival is poor in uremic diabetics [40,41], who have the highest rate of mortality on the waiting list for transplantation [8,42]. Therefore, in most circumstances, transplanting a pancreas along with a kidney is justified in uremic patients with insulin-dependent diabetes, whether type 1 or 2. In addition, a functioning pancreas transplant prevents recurrence of disease in a functioning kidney transplant in the long run, by providing a milieu of normoglycemia. It is worthwhile to note that according to the American Diabetes Association, 30–40% of patients with type-1 or 2 diabetes progress to end-stage renal disease, and that diabetic nephropathy is currently the most common cause of end-stage renal disease, accounting for about 40% of newly diagnosed patients. Given the significantly larger number of type-2 diabetics in comparison to type-1, the former account for nearly half of diabetics requiring dialysis [8].
The recipient selection criteria for pancreas-transplant patients aged 18–65 years are an ability to withstand surgery and immunosuppression, psychosocial stability, social support, compliance and a commitment to long-term follow-up, the presence of secondary complications of diabetes or hyperlability (“complicated diabetes”), and the absence of any exclusion criteria. The exclusion criteria include insufficient cardiovascular reserve, coronary angiographic evidence of uncorrectable coronary artery disease or ejection fraction below 30%, recent myocardial infarction, ongoing substance abuse (drug or alcohol), major ongoing psychiatric illness, recent noncompliance, lack of social support, active infection or recent malignancy, lack of well-defined diabetic complications, and significant obesity (BMI >30–35 kg/m2).
Renal replacement therapy in diabetics
Studies have shown that there is a survival benefit for SPK versus deceased- (and possibly living-) donor kidney transplant alone, and for SPK and PAK transplants (and possibly PTA) verus remaining on the waiting list [43–47]. In selected diabetic patients, adding a simultaneous pancreas to a kidney transplant results in improvement of the results of pancreas transplantation and does not adversely affect either patient or kidney graft survival. Apart from being an acutely life-enhancing modality, SPK transplant is also life-prolonging.
Expansion of the donor pool
Due to a continued organ shortage and an increase in diabetics as well as in diabetic nephropathy, continued inroads are being made into increasing the existing donor pool. The main areas have been the use of donation after cardiac death (or DCD), the use of older donors, and the use of segmental grafts from living donors.
DCD donors
There has been a significant increase in the number of pancreata transplanted from DCD (or asystolic) donors over the last few years [5,38,48–51]. The reported outcomes from these donors have been shown to be excellent, but more stringent criteria are imposed on them to ensure that they are relatively younger and have no other significant comorbidity, and that the cold-ischemia times are lower [50]. Recently, some authors have reported the use of extracorporeal interval support for organ retrieval (EISOR) for renal and extra-renal organ procurement [38]. Despite having small numbers in the study group, they have shown 100% patient and pancreas graft survival, at least on short-term follow-up, as well as rates of delayed graft function in the kidneys significantly lower than those seen in kidneys transplanted from conventional DCD donors. The process entails pre-mortem cannulae placement, with the consent of donor family members, prior to withdrawal of life support. After declaration of death, and a requisite wait of 5 minutes of cardiac arrest, the organs are perfused by starting cold perfusion, and the procurement is performed in a controlled fashion. However, potential problems of cardiac reanimation and ongoing cerebral flow with EISOR may be an issue, and this process has not yet gained widespread acceptance, given the ethical and sensitivity issues involved. In another study [38], the authors compared the outcomes of an extended donor-criteria pancreas transplant cohort of SPKs (which were defined as age ≥ 45 years or DCD) with SPKs from conventional donors, and given a similar median follow-up of 29 months, patient, kidney, and pancreatic graft survival rates, delayed graft-function rates, rejections and infections, and surgical complication rates were similar. The 1-year mean serum creatinine or glycohemoglobin were also similar between the two groups.
Solid-organ pancreas transplants from living donors
Pancreas transplants have been performed in the past using living donors (Figure 5.3) and the long-term graft survival rate is reported to be significantly higher for living- (versus deceased-) donor pancreas transplant recipients [52]. The first living-donor segmental pancreas transplant was done at the University of Minnesota in 1979, and insulin-independence was achieved [53–56]. There have since been more than 150 cases reported worldwide (124 reported from the University of Minnesota) [57]. In the Minnesota experience [55,57,58], the reason for choosing a living donor, initially for solitary pancreas transplants (PAK, PTA), was due to the high rejection and low survival rates seen with pancreata from deceased-donor grafts, along with a shortage of deceased-donor grafts. The complications reported in the recipients of living-donor SPKs included thrombosis in 3%, pancreatic enzyme leak in 5%, bleeding in 4%, abscess formation in 7%, and infected aneurysm in 1%. The pancreas technical failure rate was 11% and the postoperative mortality was nil. The incidence of development of diabetes in the donors by formal studies was about 30%, and 6% were on insulin. However, with the application of stricter preselection criteria, the insulin-dependent diabetes risk in the donor was reduced to 1%.
Potential donors may undergo either segmental pancreas donation alone (for non-uremic or post-uremic recipients) or simultaneous segmental pancreas and unilateral kidney donation (for uremic recipients). Once identified, potential donors should be subjected to a thorough medical, metabolic, and psychosocial screening. ABO and human leukocyte antigen (HLA) cross-match compatibility is preferred but not mandatory. A segmental donor pancreatectomy can also be applied for islet isolation and allotransplantation, if facilities for islet-cell isolation are available.
Expansion of the recipient pool
An International Pancreas Transplant Registry (IPTR) analysis [1,5] shows that there has been an increase in pancreas transplants implanted into diabetic uremic recipients with measureable C peptide (or type-2 diabetics), older donors, African-American ethnicity, pancreatic retransplants, major HLA locus mismatch, and HLA sensitization.
Transplanting type-2 diabetics
There has been a paradigm shift in the approach towards selecting diabetic uremic candidates for transplantation. Traditional criteria for accepting recipients for pancreas transplantation required type-1 diabetics [59]. Transplantation in patients who are C-peptide positive, or who are type-2 diabetics, is a topic of ongoing discussion. Individuals belonging to this subset are usually older, obese, of African descent, and have peripheral insulin resistance. But the common thought that they cannot benefit from a pancreas transplant has been challenged by several studies, and currently most of the available evidence suggests that they do indeed benefit and become insulin-independent [37,39,60]. A study done in the University of Minnesota [60] showed that pancreas transplants can provide excellent glucose control in recipients with type-2 diabetes. All 16 of their recipients whose transplant was technically successful (94%) were rendered euglycemic. Long-term results were comparable with those seen in transplant recipients with type-1 diabetes. A study from Wake Forest University [37] compared outcomes between patients with absent or low pretransplant C-peptide levels (2.0 ng/ml) and those with levels of 2.0 ng/ml who received an SPK transplant, and given a similar median follow-up of 40 months the death-censored kidney and pancreas graft survival rates were similar, but patient survival was slightly lower in the C-peptide-positive recipients. The latter were older, showed a later age of onset of diabetes, weighed more, included a greater proportion of African-Americans, and had a longer pretransplant duration of dialysis. It is speculated that the increase in β-cell mass helps overcome insulin resistance or challenges the theory of insulin resistance. However, it has to be taken into account that the procedural and postoperative complications could be higher in these individuals due to their obesity and cardiac status. It has also been observed that they have continued need for oral hypoglycemic agents, despite being insulin-free [37].
Currently approved organ allocation policy in the USA (pending implementation) allows allocation of kidney–pancreata and pancreata to insulin-dependent, non-obese, C-peptide-positive recipients.
Therefore, to summarize, the current evidence suggests that the main consideration for SPK and PAK is the surgical risk, and the main consideration for PTA is hypoglycemia unawareness. There is little reason not to do an SPK or PAK transplant in non-obese insulin-dependent type-2 diabetics. PTA is largely limited to hypoglycemia unawareness and nearly all patients are C-peptide-negative. If a true hypoglycemia-unaware C-peptide-positive PTA candidate exists, the transplant should be done. The C peptide is of importance only in that hypoglycemia unawareness is rare if positive.
Recipient pretransplant work-up
Pretransplant work-up should include a detailed history, cardiopulmonary risk assessment, and vascular examination. Most transplant candidates may require coronary angiograms, since noninvasive tests such as myoview scan are not good predictors of cardiac risk in long-standing diabetes [61,62]. In healthy young subjects, dobutamine stress echocardiograms may suffice [63]. If coronary artery disease is detected on angiograms, angioplasty/stenting or surgical revascularization is indicated, and such patients have a lower rate of postoperative cardiac complications compared to those with medical therapy alone [64]. Assessment of the iliac vasculature is important, usually using a duplex scan.
Choice of operation: SPK/PAK/PTA
The three common types of operation performed for pancreas transplant are SPK, PAK, and PTA. These techniques have been described and evolved based on experience [65–69]. According to the latest IPTR report [5], of the 35 000 pancreas transplants performed worldwide that were reported, 75% were SPK, 18% were PAK, and 7% were PTA. Pancreas transplantation usually involves placement of the pancreas intraperitoneally, although some centers are implanting in an extraperitoneal position[69]. PTA is performed for hypogycaemia unawareness, as described earlier.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree