Introduction
Type-1 diabetes mellitus (T1DM) affects millions of people worldwide and the incidence is rising. The discovery of insulin in 1922 meant that the acute symptoms of T1DM were able to be successfully treated with insulin injections and it is now rarely fatal. However, the chronic, secondary complications of the disease, including neuropathy, retinopathy, and both micro- and macroangiopathy, are now associated with a high personal cost for people with T1DM, including blindness, renal failure, ischemic heart disease, and peripheral vascular disease, as well as an enormous economical cost for Western societies. Novel T1DM therapies therefore aim to stabilize or reverse these secondary complications, or ultimately to prevent these complications altogether by fully reversing T1DM early in the disease. There are a number of potential ways of achieving this, but pancreatic islet transplantation has several key advantages over alternative therapies.
The aim of this chapter is to give an overview of islet allotransplantation, including an outline of: (i) the rationale for this treatment, (ii) the selection and allocation of suitable donor pancreases, (iii) pancreas retrieval and preservation, (iv) human islet isolation, (v) islet culture, (vi) pretransplant graft assessment, (vii) islet recipient selection, (viii) the islet transplant procedure, (ix) post-transplant management, (x) immunosuppression, (xi) the current clinical outcomes, and finally (xii) the ongoing challenges facing the field.
Rationale for islet transplantation
Although the development of the chronic complications of T1DM is multifactorial, there is clear evidence that they are closely linked to the tight glycaemic control. In addition to observations made in studies involving identical twins, studies such as the Diabetes Control and Complications Trial (DCCT) have elegantly demonstrated that intensive insulin regimens resulting in tight glycemic control can significantly reduce the incidence of secondary complications of diabetes compared with conventional insulin treatment [1]. However, the intensive regimens are also associated with life-threatening hypoglycemia [2]. The challenge, therefore, is to develop treatments that are well tolerated, and that ensure restoration of normal glucose homeostasis at an early stage of the disease (i.e. treatments that truly reverse T1DM), in order to prevent the secondary complications from developing, rather than trying to treat these severe conditions once the “horse has already bolted”. In addition, as T1DM is principally diagnosed in the first 2 decades of life, any candidate treatment needs to be applicable to children and young adults.
There are a number of potential ways of achieving tight glycemic control. Some of the severe drawbacks of intensive insulin injections can be improved by using insulin pumps, especially if these can be combined with glucose sensors [3,4]. However, this approach still has the disadvantages that it uses exogenous insulin and a mechanical device and that conceptually it is still treating diabetes, rather than truly curing it. The latter can, however, be achieved by the transplantation of insulin-producing tissue, in the form of either a vascularized pancreas transplant or a pancreatic islet cell transplant (ICT). Whole-pancreas transplantation is discussed in detail in Chapter 5, and is a very successful treatment that achieves restoration of normal glucose homeostasis and results in insulin independence rates of up to 85% 1 year post-transplantation. In addition, there is growing evidence that this treatment also reverses the secondary complications of T1DM [5]. However, whole-pancreas transplantation is intrinsically a major surgical procedure, with a high procedure-related morbidity, and mortality rates of up to 4%. The major complications include graft thrombosis, graft pancreatitis, pancreatic fistulae, and pseudocyst formation, which are related to the pancreatic exocrine tissue rather than the β-cell component. Islet transplantation, on the other hand, is a minimally invasive procedure with the same potential to restore normal glucose homeostasis. However, by transplanting only the endocrine component of the pancreas (about 2% of the pancreas mass) as a cellular transplant, this procedure is associated with a very low rate of serious complications. In addition, cellular transplants have the huge potential advantage of being able to be immunomodulated or immunoisolated, thereby preventing the need for long-term immunosuppression (see later). Islet transplantation is potentially widely applicable to children and teenagers. The advantages and disadvantages of whole-pancreas and islet transplantation are compared in Table 6.1.
Table 6.1 Characteristics of islet and pancreas transplantation as modalities of β-cell replacement therapy.
| Islet transplant | Pancreas transplant | |
| First performed | 1974 (Minneapolis) | 1966 (Minneapolis) |
| Total number of cases | >1,400 | >24,000 |
| Donor : recipient ratio | 1 : 1–4 : 1 | 1 : 1 |
| Number of transplants required to achieve therapeutic targets | 1–4 | 1–2 |
| Pretransplant graft testing | Extensive testing possible | No means at present |
| Preferred mode | IA | SPK |
| Transplantation procedure | Percutaneous | Laparotomy |
| Amount of tissue transplanted | 0.5–5.0 g | ∼100 g |
| Procedure-related complications | Minimal:
| Significant:
|
| Mortality risk | Negligible (∼0%) | Moderate (4%) |
| Insulin independence | ||
| 1 year | 75% | 85% |
| 5 years | 15% (<60%) | ∼70% |
| Graft function at 5 years | 70% | 70% |
IA, islet alone; SPK, simultaneous pancreas-kidney.
It is important to emphasize that whole-pancreas transplantation and islet transplantation should be regarded as complementary rather than competitive therapies, and as such, allocation of patients to these two treatments should be tailored to individual patient need. In addition, although the ultimate goal of islet transplantation is to achieve long-term insulin independence, currently the main indication for this treatment in most countries is to treat hypoglycemic unawareness after all conventional treatments have been exhausted. It is extremely effective at reversing this life-threatening complication of T1DM. However, it must not be forgotten that the potential application of islet transplantation is much greater.
Historical background of islet transplantation
The first attempt to cure diabetes using β-cell replacement was performed in Bristol in 1883, when minced pancreas procured from a sheep was injected into a patient with T1DM [6]. This attempt failed, and with our current knowledge of transplant immunology, this is not surprising! In 1916 the first pancreatic tissue allograft was performed in Newcastle-upon-Tyne by Dr Pybus. In his report, published in The Lancet in September 1924, he reported a small reduction in urinary excretion of glucose in one of two diabetic patients whom he transplanted with fragments of pancreatic tissue obtained from human cadaveric donor [7]. Once the association between islets of Langerhans and insulin secretion had been established in the 1920s, scientists started isolating islets from animals in order to study islet physiology, with the hope of treating and curing diabetes. Initially, islet isolation was performed using mechanical disruption, microsurgical dissection, and hand-picking. However, in 1965 Moskalewski developed a new technique of tissue dissociation using a crude preparation of enzymes obtained from culture supernatants from the bacterium Clostridium histolyticum and successfully isolated pancreatic islets from a guinea pig [8]. This technique was quickly adopted by Paul Lacy and Mary Kostianovsky in order to isolate islets from rats [9]. It was not until 1974, however, that the first clinical islet allotransplant using isolated islets of Langerhans was performed [10].
Improvements in both islet isolation and islet transplantation occurred over the next few decades, including the development of a semiautomated method for pancreas digestion by Ricordi and the introduction of a large-scale method for islet purification by Lake [11]. However, despite the fact that 494 islet transplants were reported to the International Islet Transplant Registry between 1974 and the end of the 1990s, and the fact that in animal models reversal of diabetes following an islet transplant was almost routine, the results of clinical islet allotransplantation were disappointing, with overall 9% of patients achieving insulin-independence and about 40% achieving partial graft function in the form of C-peptide production (C-peptide levels >0.3 ng/ml) [12].
However, all this changed in 2000 when Shapiro published the results of a small series of islet transplants performed in Edmonton, Canada [13]. Using a steroid-free immunosuppression regime, together with a protocol that involved each patient receiving at least two consecutive islet transplants utilizing large islet numbers, he reported that all seven of his patients had achieved insulin-independence. In addition, the main indication for transplantation in this group was the severe complication of hypoglycemic unawareness, which was also universally reversed by the islet transplants. This landmark publication stimulated the reactivation of other islet-transplant centers across the world, as well as the creation of many new centers. Soon leading groups were reporting insulin-independence rates of 80–85% at 1 year, success rates that were comparable with whole-pancreas transplantation [14].
Donor selection and pancreas allocation
Islet transplantation involves two broad components, namely islet isolation and the islet transplant itself. One of the major challenges of clinical islet transplantation has been to consistently obtain sufficient numbers of high-quality islets to routinely achieve insulin independence after transplantation. There are a number of factors which affect islet-isolation outcome, including donor selection, the variability of pancreas digestion, and the recovery of islet function after isolation and transplantation [15–17].
As with all other forms of allotransplantation, islet transplantation is hindered worldwide by the shortage of organ donors. However, this is compounded by the fact that whole-pancreas teams and islet-transplant teams are principally competing for the same donor organs [18]. Although it is widely stated that the donor criteria for the two modalities are distinctly different, this is based on the criteria of optimal islet yields (islet yields are expressed as both islet numbers and a volume-adjusted islet equivalent (IEq)) rather than the more important measures of pre- and post-transplant islet function. This paradox is exemplified by donor age. Ihm et al. have shown nicely that although islet yields increase with donor age, islet function as measured by insulin secretion decreases with age [19]. Therefore, although older donors (>60 years old) are largely excluded from whole-pancreas transplant programs and would be readily available for islet transplants, the reduced islet function and increased apoptosis rates within the islets of these donors mean that they are also largely unsuitable for islet transplantation. There are, however, a few donor factors that favor retrieval for islet transplantation over whole-pancreas transplantation, including high donor body mass index (BMI). These pancreases are often associated with poor vasculature for anastamosing a whole-pancreas graft, but provided the donor has not developed type-2 diabetes, isolated islets from obese donors can have excellent in vitro and in vivo function. The challenge for all forms of transplantation is to be able to expand the donor pool, including finding methods of enabling marginal donors to be used. Table 6.2 summarizes current exclusion criteria for pancreas donors for clinical islet isolation and transplantation in the UK.
Table 6.2 UK exclusion criteria for pancreas donors for clinical islet isolation and transplantation. DBD, donation after brain death; DCD, donation after cardiac death; DSA, donor-specific antibody; ICU, intensive care unit.
| Donor criteria |
Absolute exclusion criteria
|
Relative exclusion criteria
|
Donor age
There are several reports demonstrating that donor age correlates positively with isolation yield and purity [16,17]. Nonetheless, it is now widely accepted that islets isolated from pancreases from donors younger than 55 years demonstrate significantly better function in vitro compared with those of older donors [19]. In addition, the expression of the pancreatic master gene Pdx1 (whose product, Pdx1, is a pancreatic transcription factor) is decreased with increasing age, while the apoptotic index increases with age. Interestingly, the outcomes of solid-organ transplantation are also better when pancreases from young donors are used. One of the challenges for islet transplantation, however, is that the current methods used for human islet isolation favor the older donor. The pancreases from young donors (<25 years) are very difficult to process, and usually do not yield sufficient numbers of islets for transplantation [15,16]. This seems to be related to the fact that the collagenase enzymes routinely used for pancreas digestion do not efficiently digest the extracellular matrix within the younger pancreas. Encouragingly, newer enzymes seem to be improving isolation outcomes from younger donors.
Body mass index
High body mass is associated with increased deposition of intra-abdominal adipose tissue and the presence of excessive peripancreatic fat. This may result in suboptimal cooling of the pancreas during retrieval and suboptimal preservation. This in turn increases the risk of complications after solid-organ transplantation, including pancreatitis and graft loss. Several studies have demonstrated that both donor mass and donor BMI correlate with islet isolation outcomes (total islet numbers, as well as IEqs) [15,16,20]. Nevertheless, islets isolated from obese donors have always been treated with some caution as the donors tend to have a type-2 diabetes phenotype. However, recent studies have shown that, provided severe fatty infiltration is not present, fully functional islets can be isolated successfully from donors with a BMI up to 40, and successfully transplanted, even if the donor’s glycated hemoglobin is above 7.0% [21].
Cold-ischemia time
Prolonged cold ischemia is detrimental to pancreatic islet isolation. Although it is well documented that cold storage times of up to 8 hours are well tolerated, there is an overall negative correlation between the storage time and islet yield and quality [15,22,23]. Although viable islets can still be isolated from pancreases stored for longer periods of time, the results are inconsistent, and there is a great reluctance to use them for clinical transplantation. A two-layer method (TLM) of pancreas storage has been developed in order to enable pancreases to be effectively stored for long periods (see next section). Although it has some limitations, many groups use this method for suboptimal pancreases with cold ischemia times exceeding 8 hours [24,25].
Pancreas retrieval and preservation
Pancreases for clinical islet isolation should be retrieved in an identical manner to that used for vascularized pancreas transplantation, with the exception of the vessel dissection [26]. The pancreas is therefore removed en bloc with the duodenum and spleen using a no-touch technique. During pancreas dissection, a meticulous technique is required, with avoidance of excessive use of diathermy. Adequate perfusion of the pancreas is of paramount importance and in situ perfusion is often complemented by additional ex vivo perfusion on the back table. During multiorgan retrieval, intravascular cooling is achieved by either aortic or aorto-portal flush. The latter has negative effects on the pancreas and should be avoided. Reduction of the pancreatic core temperature is considered one of the most important steps during pancreas retrieval [27]. Intravascular flush with cold preservation fluid is usually not sufficient and needs to be complemented by external pancreas cooling using copious amounts of ice slush placed in the lesser sac.
Standard preservation techniques involve simple cold storage at 4 °C in University of Wisconsin solution (UW), although some centers use HTK, Celsior, EuroCollins, and Kyoto solutions [28,29]. The pancreas is immersed in at least 300 ml of preservation solution and double-bagged. These conditions allow for preservation of the pancreas for up to 8 hours, with minimal loss of organ quality.
Other preservation protocols have been developed, including the TLM, in which the pancreas is preserved in hypothermia on the interface between oxygen-charged perfluorocarbon and preservation fluid.
Although recent papers have questioned the validity of the TLM, showing very limited tissue penetration of oxygen and of the perfluorocarbon itself [30–32], the 2009 Collaborative Islet Transplant Registry (CITR) report revealed that up to 33% of pancreases retrieved for islet isolation were preserved using this method. Examples of experimental preservation techniques include persufflation of pancreases with gaseous oxygen [33,34] and continuous and pulsatile hypothermic pump perfusion [35]. Thus far, none of these techniques has been used clinically, although recently presented results are encouraging.
Islet isolation procedure
Although groups have been undertaking islet isolation for many years [36–39], the human islet isolation procedure is still far from optimized, providing transplantable yields of islets in less than 50% of pancreas preparations, even in centers with significant experience. The main goal of islet isolation is to extract viable islets that are free from surrounding acinar tissue.
Most centers worldwide use a modified semiautomated technique for human islet isolation described by Ricordi [37]. This involves a two-stage procedure. First, the pancreas digestion stage involves the pancreas being dissociated by a combination of mechanical and enzymatic breakdown, resulting in the islets being liberated from the surrounding exocrine tissue. During this stage, the pancreas is transferred to the isolation facility, where it is dissected and the duodenum, spleen, and superficial fat are removed. The pancreatic duct is identified and cannulated with a venous catheter, and commercially available bacterial collagenase dissolved in Hanks’ solution at 4 °C is delivered to the pancreatic parenchyma by intraductal injection, either by a hand-held syringe or by recirculating pumps. The distended pancreas is then divided into a number of pieces and transferred into a metallic or polycarbonate chamber containing marbles or ball bearings. This chamber is connected to a fluid-filled system, in which a medium (usually minimal essential medium, MEM) is circulated at 37 °C. This system allows for accurate control of temperature and fluid flow, which can both be adjusted depending on the progress of digestion. When free islets appear in the digest, the process is stopped by addition of cold buffer into the circuit, and islets are collected into an albumin-enriched medium, commonly containing a cocktail of protease inhibitors.
In the second stage of islet isolation, islet purification, the liberated islets are separated from the exocrine tissue using centrifugation on a density gradient [39–41]. Following incubation of the pancreatic digest for 30–60 minutes in UW, resuspended tissue is loaded on the top of a Ficoll-based continuous gradient within a spinning COBE 2991 cell separator. During centrifugation, tissue fragments and cellular debris accumulate in the top layer (lowest density), big fragments of tissue move towards the bottom of the gradient (highest density), and liberated islets stay in between the two extremes (1.08–1.09 g/l). Purified islets can be transplanted immediately or following a short-term culture.
In order to comply with recent European Union (EU) and US Food and Drug Administration (FDA) regulations, human islet isolation for allotransplantation must now be performed in purpose-built good manufacturing practice (GMP)-grade facilities. As a result, an increasing number of islet transplant networks is developing, in which several centers transplant islets isolated from one core facility. This “hub and spoke” model not only means that the costs can be rationalized, but enables islet-isolation expertise to be centralized and maintained [42].
Islet culture
Although the original Edmonton protocol emphasized the importance of transplanting freshly isolated islets, most groups now recommend a period of 24–48 hours of culture prior to transplantation. Pretransplant culture of clinical preparations has two main purposes. First, it allows for a more thorough assessment of graft quality before the final decision to transplant [43]. Often an islet preparation can look excellent immediately after islet isolation, but a period of culture can show that in fact cell death was commencing, and after 24 hours of optimal culture the islets may become fragmented and have reduced function. This is particularly the case with large volume islets, in which central necrosis may not be evident immediately [44,45]. It is clearly beneficial to discover this before rather than after the islet transplant. On the other hand, there is clear evidence that a period in culture can also enable islet function to improve, as islets recover from the islet isolation procedure [46]. Second, the logistics of transplanting fresh islets used to mean that patients often had to be admitted in the middle of the night, and the procedure itself became an emergency. A period of islet culture enables the procedure to become semielective and carefully planned.
The specifics of different culture protocols are beyond the scope of this book. However, multiple measures are undertaken in order to limit the damage caused by hypoxic conditions, including low islet seeding density, low depth of medium to facilitate oxygen diffusion, and a wide range of supplements to reduce production and release of pro-inflammatory cytokines and free radicals.
In many centers, gas-permeable bags (similar to the bags used for platelet storage) have been used to facilitate oxygen diffusion to islets in culture. The purification process ends with several fractions containing different degrees of contamination with exocrine tissue. It is a common practice to culture high- and low-purity fractions separately. Purified tissue is placed in large culture flasks (175 cm2 cultivation area), usually in cell-culture medium, such as CMRL-1066. The seeding density is about 20 000–30 000 IEq in 25–30 ml of culture medium. This seeding density, combined with a low depth of culture medium, helps prevent hypoxia in cultured islets. At the end of the culture period, the preparation is reassessed (islet number/loss, viability, microbiological status) and its suitability for transplantation is reevaluated. Although technically possible, prolonged culture is avoided in clinical transplantation and limited to a maximum of 48 hours.
Pretransplant graft assessment
Pretransplant culture of isolated islets allows for assessment of graft quality in terms of morphology, functional status, and sterility. This reduces the chance of poor-quality or contaminated grafts from being transplanted and makes this treatment modality potentially safer than solid organ transplantation. Several parameters are assessed during this process [47–49].
Islet number, size distribution, and islet morphology
Although automated counting is an option, most centers count islets manually [49–51]. This process, while fairly robust, is operator-dependent and the results can differ significantly among centers. In order to identify islets in a suspension, a zinc chelator—dimethylthiocarbazone (Dithizone, DTZ)—is used, which stains islets crimson red [52]. The size distribution of an islet preparation is usually skewed towards small islets; therefore, islet number is not necessarily representative of β-cell volume. In order to adjust for this discrepancy, IEq is used, where 1 IEq represents an islet 150 µm in diameter [53]. The whole population of islets is stratified into size-related groups, with corresponding conversion factors. For instance, for an islet 50–100 µm in diameter, this conversion factor is 0.167, and for an islet 200–250 µm in diameter, it is 3.5. In addition to the number of IEqs, an isolation index (II) is calculated by dividing the number of IEqs by the number of islets. II provides an indication of whether the distribution is skewed towards small or large islets. Analysis of islet morphology determines the degree of fragmentation and cleavage of peri-islet exocrine tissue.
Purity of the preparation
The purity of the preparation is estimated as the percentage of islets compared to acinar tissue. It is subjective, with considerable interassessor variability, but a purity of >50% is required for an islet preparation to be signed off for transplantation in the UK. Indeed, it could be argued that a preparation of <50% is by definition an exocrine transplant!
Islet viability and function
For the purpose of clinical isolation, a fluorescent viability assay is performed [54,55]. This assesses the integrity of the cellular membrane, which in turn is a function of the cell’s metabolic state. A principle of this viability test is that dyes such as ethidium bromide or propidium iodide can diffuse into cells and bind to DNA only if cellular-membrane permeability is compromised, as in dead cells. Others, such as fluorescein diacetate or acridine orange, are actively transported inside an intact, living cell and can be either metabolized, resulting in release of fluorescent compound, or bound to DNA. The assessment of islets is performed manually using a fluorescent microscope and is again subject to operator bias.
A glucose-stimulated insulin secretion (GSIS) assay tests the ability of isolated islets to response to glucose stimulation by insulin secretion. Several modifications of this assay exist, but for clinical purposes a static incubation is usually performed, whereby islets are exposed to a baseline (2.5 mM) and/or a stimulatory (25.0 mM) glucose concentration. Following a period of incubation, the insulin concentration before and after stimulation is measured, and the stimulation index (SI) is calculated using the formula:
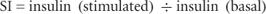
where insulin (stimulated) is the amount of insulin secreted during 1-hour incubation in 25.0 mM glucose medium and insulin (basal) is the amount of insulin secreted during 1-hour incubation in 2.5 mM glucose medium.
An SI in excess of 2 is indicative of a functional preparation. Although this test is used to assess the islet graft, it is usually performed after transplantation. Therefore, its results are not part of the product release criteria listed later.
Microbiological contamination and the presence of endotoxin
Culture of isolated islet allows for a thorough microbiological assessment of the graft prior to transplantation [56,57]. Routine microbiological tests prior to graft release include microscopy and Gram staining, endotoxin levels, and bacterial and fungal cultures. As part of microbiological monitoring, the donor’s viral status should be included with product release documentation (cytomegalovirus (CMV) and Epstein–Barr virus (EBV) status in particular) so that the transplanting team can consider appropriate prophylaxis [58,59].
Product release criteria
The current regulations for tissue banks in Europe dictate that clear product release criteria have to be determined and stuck to. In the UK, the following release criteria are used:
- Islet yield >200 000 IEq (although the graft is allocated based on the islet dose in IEq/kg).
- Purity >50% (>30% in the USA).
- Viability >70%.
- Packed cell volume (PCV) <10 ml (recent research from Edmonton suggests that PCV should be limited to 5 ml in order to eliminate the risk of portal-vein thrombosis [60]).
- Microscopy and Gram stain negative.
- Endotoxin level <3-5 EU/ml (values differ between manufacturers and isolation centers).
Islet recipient selection
As islet transplantation develops as a successful treatment, the indications expand and more patients become eligible for its benefits [61]. Overall, however, there are two main groups that are included in the selection criteria worldwide. First and foremost is the small subpopulation of patients suffering from brittle type-1 diabetes, with severe life-threatening hypoglycemic unawareness. These patients benefit enormously from the minimally invasive islet transplants, but are only offered an islet transplant provided that all optimal insulin treatments have been exhausted. The second group is patients with unstable type-1 diabetes in whom a renal graft must be preserved [62]. These transplants can either be performed as a simultaneous islet and kidney transplant (SIK) or as an islet-after-kidney graft (IAK).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








