Introduction
The long-term outcomes for children with end-stage renal failure (ESRF) have improved over the last few decades with the development of specialist pediatric nephrology and renal-transplantation services throughout the world. This requires a dedicated multidisciplinary team of pediatric nephrologists, as well as general and other subspecialist pediatricians, pediatric renal-transplantation, urological, and general surgeons, specialist nurses, pharmacists, and dietitians, plus additional allied-health professionals and psychosocial team members. In the past, when children were born anuric in nonobstructive ESRF, the only medical option was to offer symptom-care therapy and allow the child to die. Nowadays, if the neonatal course is uneventful with good cardiorespiratory status, dialysis can be offered as a bridge to renal transplantation in the second year of life (and in some circumstances, earlier). If a child has a good functioning renal allograft with minimal complications, this can give an excellent quality of life. However, it is not always possible to predict which children are going to do well, with good physical, psychological, growth, and pubertal development.
The intensive management of children with ESRF is complex and practice varies around the world. The causes of ESRF are different and the long-term outcome, with multiple transplantations in the future, has to be considered. This chapter will focus on the differences between children and adults with respect to the causes of ESRF, pretransplantation work-up, peritransplantation management, and the subsequent course after transplantation.
Pediatric ESRF
One of the most powerful moments in an adult’s life is seeing their unborn child for the first time on an ultrasound. This potentially joyous occasion can be devastating for parents if there is evidence of significant fetal renal disease. Antenatal ultrasound screening has completely transformed perinatal nephro-urological practice through identification of fetuses with significant renal abnormalities who may be born with severe kidney and/or respiratory failure [1]. However, practice varies throughout the world, with routine antenatal ultrasound scanning occurring at various times throughout the pregnancy, but usually at least at 20-weeks’ gestation, when most abnormalities will be identified. In addition, many units in the UK offer early scanning at 12-weeks’ gestation, with some countries also offering routine third-trimester ultrasound scanning. Antenatal ultrasound can identify other severe organ abnormalities, allowing a discussion of potential severe comorbidities associated with poorer prognosis. This allows time to counsel parents in the options, which may include termination, antenatal intervention (such as vesicoamniotic shunting), and planned intervention (such as being born in a unit with a link to pediatric nephrological center). However, despite antenatal ultrasound screening, some cases of renal abnormality are missed until a child presents with symptoms and/or signs of chronic kidney disease (CKD) or ESRF. Some cases, such as congenital nephrotic syndrome, may have no detected abnormalities until birth (although they may be diagnosed antenatally by elevated maternal alfafetoprotein levels and/or via a previous affected child by prenatal genetic diagnosis). There may be a delay in diagnosis until the neonate or infant presents with massive proteinuria, hypoalbuminemia, and resultant edema.
The causes of childhood ESRF differ from those in adults, who usually have diabetes mellitus, hypertension, or glomerular disease. The commonest cause of CKD and ESRF is congenital abnormalities of the kidneys and urinary tract (CAKUT; Figure 9.1). This usually manifests as congenitally malformed kidneys with bilateral renal dysplasia, which may be associated with vesico-ureteric reflux and/or obstructive uropathy—namely posterior urethral valves in boys. There needs to be full urological and transplant-surgical consideration for pretransplant assessment in this group of patients [2]. Many cases of CAKUT will be caused by known genetic mutations, but at present these only account for 15% of cases in cohorts of nonsyndromic patients with renal hypodysplasia [3]. Children with congenital abnormalities may present with ESRF at any age, from antenatally to birth to adolescence. The most common genetic cause of ESRF within the first 2 decades of life is one of the hereditary cystic renal diseases, called juvenile nephronophthisis [4].
Figure 9.1 Causes of ESRF in childhood. Data from the 11th annual United Kingdom Renal Registry pediatric end-stage renal failure report [43]
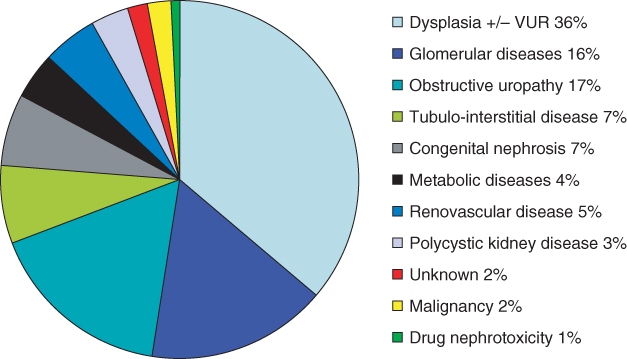
There is increased glomerular disease in children with ESRF, with the commonest cause being steroid-resistant nephrotic syndrome secondary to the histopathological diagnosis of primary focal and segmental glomerulosclerosis; this may also be resistant to other immunosuppressive drugs and has a 30–50% chance of recurring in the renal allograft. All children should have full genetic studies before embarking on renal transplantation, in order to try and evaluate the risk of recurrence post-transplantation, which may impact on the decision regarding identification of possible living related or deceased donors.
Over the last decade, increasing consideration has been given to children with ESRF who also have multisystem and metabolic conditions, some of whom have multiorgan transplant requirements. Options include simultaneous pancreas and kidney transplantation (usually for adults with type-I insulin-dependent diabetes mellitus, but also considered for children with hemolytic uremic syndrome who develop insulin-dependent diabetes mellitus) and possibly combined or sequential liver–kidney transplantation for primary hyperoxaluria type 1 [5]. There is also an increasing epidemic of CKD and ESRF caused by calcineurin nephrotoxicity from other solid-organ transplantation, such as cardiac, lung, and lung–cardiac transplantation [6].
Pretransplantation work-up
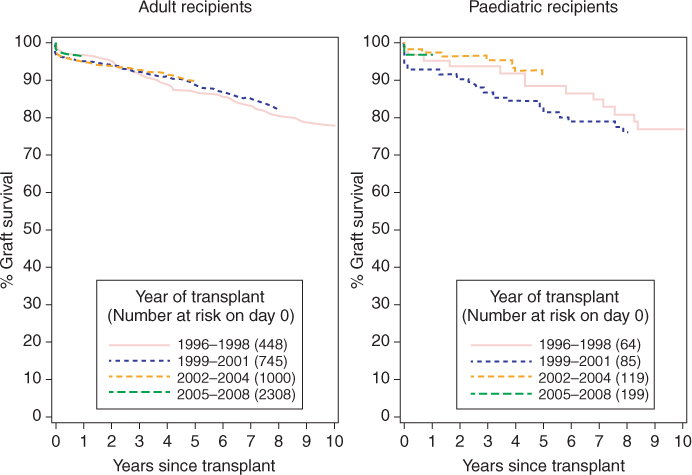
The patient and renal allograft survival rate for children receiving renal transplantation for ESRF has improved over the last decade and is comparable to that in adults (Figure 9.2). The pretransplantation work-up requires a multidisciplinary-team approach, involving physicians (pediatricians, pediatric nephrologists, and other subspecialists; anesthetists; intensivists), surgeons (pediatric surgeons; urologists; transplant surgeons) and specialized nurses, pharmacists, dietitians, psychologists, and other members of the psychosocial team.
Figure 9.2 Ten-year renal allograft survival from first living-donor renal transplantation in adult and pediatric recipients in the UK. (Data supplied and reproduced with permission from National Health Service Blood and Transplant (NHSBT))
Most pediatric renal-transplantation centers state a minimum weight of 10 kg before transplantation (but that excludes consideration of fluid status and the possible increased weight of native organs, and in fact length (which would give an indication of the intra-abdominal vessel size) would be more appropriate).
The views of pediatric renal-transplantation units around the world vary, but most aim for the gold standard of preemptive living related renal transplantation where possible [7]—with the closest immunological match—as the focus is on quality and quantity of life. It is important to note that children will probably require multiple renal transplants during their lifetime. Some units wait for the requirement for dialysis before listing for deceased-donor renal transplants and exclude mismatched parental antigens (which can avoid subsequent sensitization), keeping the option of living related transplantation for the second renal transplant. However, the best long-term outcomes are for transplantation as opposed to dialysis, and living-donor renal-transplantation outcomes are better than outcomes from deceased donors, particularly when dialysis can be avoided [7].
The aim of the pretransplantation work-up is to try and plan for the best outcome for the prospective renal-transplant recipient, with individualized care according to risk stratification. This allows time for full vaccination, ensuring that all patients have immunity to potential preventable infectious complications before being transplanted. All prospective pediatric renal-transplant recipients should have the routine vaccination schedule (including diphtheria, tetanus, pertussis, polio, hemophilus influenzae, meningococcal, pneumococcal, measles, mumps, and rubella), BCG vaccination (Mantoux testing first in older children), hepatitis B (when hepatitis B surface-antigen-negative), varicella (when varicella immunoglobulin G (IgG)-negative), and repeat measles vaccination (where measles IgG-negative).
Pediatric recipients are at increased risk of early renal-allograft thrombosis due to lower renal perfusion pressures, especially small children, and a full prothrombotic screening should be undertaken if there is a personal and family history of thrombosis; anticoagulation policies should be adapted according to the results. All patients require cardiological assessment (with electrocardiography and echocardiography) as well as bladder, renal, and abdominal-vessel ultrasound screening. Patients with hostile bladders require full bladder function and urological assessment, which will typically involve urodynamic assessment of flow rate, volumes, and emptying [2]. A number of options are available to ensure that the bladder is safe for transplantation, including urinary diversion, a drainage procedure such as a Mitrofanoff, and bladder augmentation. There are currently no clearly accepted urodynamic criteria for intervention, and few studies have been performed. Combined management of these children in centers with adequate urological and transplant expertise is essential; if the bladder does not empty adequately, drainage will be required, and a small, poorly compliant bladder will probably require augmentation. The timing of such procedures in relation to transplantation is also uncertain, although there is some evidence that simultaneous transplantation and bladder surgery may lead to more complications [2]. Patients with abnormal or uncertain abdominal-vessel ultrasound screening should have magnetic resonance venography (MRV) performed [8]. Patients with increased risk of developing new-onset diabetes after transplantation (NODAT) (such as those with cystinosis) require full assessment. All patients require pretransplantation assessment by a pediatric transplantation physician, surgeon, and anesthetist.
Living-donor renal transplantation
Living-donor renal transplantation can be performed as a planned event when both donor and recipient are known to be healthy. It has improved patient and renal allograft survival, with reduced morbidity and acute rejection rates. However, there is a donor morbidity and mortality risk of 15 and 0.03%, respectively—with shorter hospital stay and less postoperative pain—after laparoscopic donation, which is now routine in most centers. There are additional aspects to consider with respect to pediatric renal transplantation when embarking on identification of possible living donors (who are usually the parents), as implantation of an adult organ into a pediatric recipient is more challenging. It may require surgery (for donor and recipient) in different hospitals, so safe and efficient transfer of organs to reduce cold-ischemia time is essential. Early postoperative communication must be available between donor and recipient. In our practice, we have used an Internet video link to facilitate this, although most of our donors are able to visit their respective recipients (in different hospitals) on the third postoperative day after laparoscopic donation. Unfortunately, around 20% of parents find they are unable to be donors due to identification of medical problems (including detection of unknown illnesses, abnormal kidneys, and solitary kidney), psychological or social issues which preclude donation, or other barriers (such as ABO incompatibility or preformed anti-human leukocyte antigen (HLA) antibodies), which may be overcome. As in adults with ESRF, unrelated donors can be considered, but improved long-term outcomes are related to minimizing HLA mismatching and subsequent sensitization, as children are likely to require retransplantation during their lifetime. Therefore, ABO-incompatible transplantation [10–13] and paired-exchange schemes should be considered for some pediatric recipients, where no living related donor is suitable.
Families should be counseled regarding the fact that children are at a higher risk of developing post-transplant lymphoproliferative disease (PTLD) due to being Epstein–Barr-virus (EBV)-naïve, while most adult donors are EBV-positive (so many children are transplanted with EBV mismatch).
In the UK, after both donor and recipient have completed their medical and psychosocial pretransplantation work-ups, the Human Tissue Authority undertakes independent assessments of all donor–recipient pairs to check suitability.
Deceased-donor renal transplantation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







