Alan J. Wein, MD, PhD (Hon), FACS, Roger R. Dmochowski, MD, FACS This chapter begins with a summary of the abnormalities of the micturition cycle produced by different types of neuromuscular disease, injury, or dysfunction. The source material for the central and peripheral factors involved in the physiology and pharmacology of lower urinary tract function (and dysfunction) are thoroughly discussed in Chapter 60. Most chronic voiding dysfunctions secondary to neurologic disease or injury are logical, meaning that they can be inferred from a knowledge of the normal physiology and pharmacology and the type(s) and location(s) of the pathologic process(es). Certain secondary factors that can modify the type of voiding dysfunction seen and that, once established, can cause persistence of a filling/storage or voiding/emptying abnormality, even after the initial precipitating factor or factors have disappeared or been corrected, are considered. The specific types of voiding dysfunction that occur secondary to the most common categories of neuromuscular disease, injury, or dysfunction are then described in detail. Ideally, in any such discussion, the expected states of the following parameters should be described (see Chapter 61 for specific definitions of terms): In Table 65–1 an attempt has been made to summarize many of these dysfunctions on the basis of the most common type of abnormal pattern that results from a given disease or injury, insofar as the parameters just listed are concerned. This abbreviated classification is not meant to be all inclusive but simply to indicate that, for the most part, an individual with a specific neurologic abnormality, and voiding dysfunction because of it, will, in general, have the type of dysfunction shown. Table 65–1 Most Common Patterns of Voiding Dysfunction Seen with Various Types of Neurologic Disease or Injury* The chapter concludes with a general consideration of the principles that should guide the selection of therapy(ies) for the dysfunctions considered. The individual therapies, and potential consequences thereof, are discussed in great detail in other chapters. The types and management of voiding dysfunction in the pediatric age group are specifically covered in Chapters 127 and 128. Key Point: Lesions above the Brainstem Key Point: Complete Spinal Cord Lesions from Spinal Cord Level T6 to S2 Key Point: Trauma or Disease below Spinal Cord Level S2 Key Point: Interruption of the Peripheral Reflex Arc What may be less obvious as a phenomenon related to plasticity are many of the changes that occur subsequent to bladder outlet obstruction. The most obvious changes that occur are those related to muscle and collagen content. However, these are themselves initiated by molecular events that ultimately cause increased contractile protein synthesis and hypertrophic bladder tissue growth (Levin et al, 1995). The initial stimulus might be stretched from overdistention (Cheng et al, 1999) or ischemia, likewise from distention (Chen et al, 1996). Compensation of the bladder smooth muscle cells to initially overcome the increased demand associated with obstruction is associated with alterations in the expression and function of many proteins involved in excitation/contraction coupling and active force generation of bladder smooth muscle (Chacko et al, 1999). Although urodynamic studies reflect obstruction, satisfactory emptying is generally preserved. Changes in the composition of the extracellular matrix occur as well, presumably also caused by an initial stretch stimulus. The ratio of type 3 collagen to type 1 collagen increases, and the localization of type 3 collagen changes as well (within and around some muscle bundle) (Macarak and Howard, 1999). The increase in connective tissue could be related to an increase in certain growth factors emanating from the smooth muscle or to a decrease in the activity of certain metabolic pathways contributing to the breakdown of various forms of collagen (Borer et al, 1999). Ischemia, which itself can be caused by obstruction or atherosclerosis, has also been hypothesized to contribute to remodeling of the extracellular matrix and fibrosis (Azadzoi et al, 1999; Mostwin et al, 2005). Bladder outlet obstruction has also been postulated to be associated with partial denervation, owing to damage to the intrinsic innervation of the bladder smooth muscle from a combination of pressure and ischemia (Turner and Brading, 1997; Mostwin et al, 2005). With all of these potential adverse changes occurring, it seems almost miraculous that the bladder can maintain its function, but it does for variable periods of time under different circumstances. However, there does come a point when the ability to fill/store and empty is adversely affected, but not necessarily to the same extent. Filling/storage changes seem related primarily to (1) changes in the extracellular matrix, leading to decreased compliance; and (2) to the appearance of phasic bladder overactivity. This overactivity could be myogenic in origin (caused by partial denervation—see Turner and Brading, 1997), or it could be neurogenic and related to another facet of plasticity. Afferent neuroplasticity mediated by nerve growth factors occurs experimentally in response to bladder outlet obstruction, a phenomenon that is inhibited by autoimmunization against nerve growth factor (Steers et al, 1996). The ability to empty can be adversely affected by factors related to neurogenic or myogenic mechanisms. The myogenic mechanisms could include a reversal of the compensatory changes that initially occur (see Chacko et al, 1999) or a breakdown of the structure and function of the proteins that enable the smooth muscle cells to take up, store, and release calcium, affecting the calcium activation of the contractile apparatus (Zderic et al, 1998; Chacko et al, 1999). Furthermore, these neurogenic changes associated with outflow obstruction may alter the neurotransmitter milieu of the lower urinary tract. In a model of fetal sheep bladder outlet obstruction, ligation of the urachus at midgestation in fetal sheep for 1 month resulted in a shifting of muscarinic, purinergic, and nitrergic mechanisms normally present during fetal development and growth. With outflow obstruction, bladder hypocontractility was induced and contractile forces decreased during stimulated conditions, consistent with denervation and the possibility of atropine resistance. Normal urothelial exerted negative inotropic effects (nitric oxide mediated) were also lost after obstruction. Additionally, loss of compliance resulted in reduced elasticity in the obstructed bladders, consistent with denervation (Thiruchelvam et al, 2003). Other evidence exists suggesting that bladder outlet obstruction alters the milieu of the lower urinary tract. Mirone and coworkers (2007) summarized changes related to bladder outlet obstruction on the ultrastructure of the bladder in prior animal models. These changes include alterations in the cytoskeleton and contractile protein matrix of the bladder, increasing expression of several growth factors and COX-2, swelling and structural destruction of the detrusor mitochondria, augmented extracellular matrix deposition with significant increase of the type III/I collagen ratio, downregulation of matrix metalloproteinases, and upregulation of tissue inhibitors of metalloproteinases, increased afferent neural activity from epithelial cells due to higher density of mechanosensitive epithelial sodium channels, and activation of normal cell and unmyelinated C fibers. All of these factors appear to be related to bladder dysfunction associated with obstruction. Thus the fact that the changes resulting from the neuroplasticity induced by these insults are permanent is not surprising. However, there are instances in which the initiating “cause” of a particular voiding dysfunction can be removed and yet the symptoms do not entirely disappear. This may be another instance in which neuroplasticity is a major factor. For instance, irritative voiding symptoms fail to disappear in a certain percent of patients with outlet obstruction who undergo surgical correction. Chai and colleagues (1998) found an increased incidence of a positive ice water test in patients with bladder outlet obstruction, indicating the presence of a primitive reflex circuitry capable of mediating an abnormal micturition reflex. Because the ice water test is mediated by C-afferent fibers, the findings support the hypothesis that bladder outlet obstruction is associated with afferent neuroplasticity, detectable in this case by ice water cystometry. Furthermore, persistence of this afferent neural plasticity after relief of the obstruction could account for at least a proportion of the symptomatic treatment failures after urodynamically successful outlet reduction. Vizzard (1999, 2000a, 2000b, 2000c, 2000d; Qiao and Vizzard, 2004) has written prolifically about various aspects of neuroplasticity, specifically on the occurrence and potential role of such changes in altered lower urinary tract dysfunction after spinal cord injury (SCI) and irritant-induced cystitis. Changes in spinal cord protein expression from retrogradely transported bladder neurotrophic factors could play a role in the neurochemical, electrophysiologic, and organizational properties of the lower urinary tract seen in both of these conditions and could account, in the latter case, for persistence of symptomatic and/or urodynamic abnormalities after the irritating stimulus is removed (e.g., in patients with interstitial cystitis). Those especially interested in this area should consult Vizzard’s articles and associated references. Cerebrovascular accident (CVA) is a common cause of death and one of the most common causes of disability in the United States and Europe. CVA is the most devastating manifestation of cerebrovascular disease, with an annual incidence in the United States that has been variably cited as approximately 550,000 (Blaivas et al, 1998a) and 83 to 160 per 100,000 (Blaivas and Chancellor, 1995a). Approximately one third are fatal, and another third require long-term nursing care (Marinkovic and Badlani, 2001). The prevalence of stroke in persons older than 65 years has been cited as approximately 60 in 1000, and in persons older than 75, 95 per 1000 (Khan et al, 1990). Wyndaele and colleagues (2005) estimate that 1 in 200 individuals will suffer from a CVA. Although CVA is the third leading cause of death in the United States (Marinkovic and Badlani, 2001), approximately 75% of stroke victims survive (Blaivas et al, 1998a) and, of these, only 10% are unimpaired, 40% have a mild residual, 40% have a significant disability, and 10% require institutionalization (Arunable and Badlani, 1993). Thrombosis, occlusion, and hemorrhage are the most common causes of stroke, leading to ischemia and infarction of variably sized areas in the brain, usually around the internal capsule. Marinkovic and Badlani (2001) cite evidence that arterial occlusion is found in 80% of patients. After the initial acute episode, urinary retention from detrusor areflexia may occur. The neurophysiology of this “cerebral shock” is unclear. After a variable degree of recovery from the neurologic lesion, a fixed deficit may become apparent over a few weeks or months. The most common long-term expression of lower urinary tract dysfunction after CVA is phasic detrusor overactivity (Wein and Barrett, 1988; Khan et al, 1990; Fowler, 1999; Wyndaele et al, 2005). Sensation is variable but is classically described as generally intact, and thus the patient has urgency and frequency with detrusor overactivity. The appropriate response is to try to inhibit the involuntary bladder contraction by forceful voluntary contraction of the striated sphincter. If this can be accomplished, only urgency and frequency result; if not, urgency with incontinence results. The exact acute and chronic incidence of any voiding dysfunction after CVA, and specifically of incontinence, is not readily discernable. The cited prevalence of urinary incontinence on hospital admission for stroke ranges from 32% to 79%, on discharge from 25% to 28%, and some months later, from 12% to 19% (Brittain et al, 1998). Sakakibara and colleagues (1999), based on their own experience and that of others, estimate that some voiding dysfunction occurs in 20% to 50% of patients with focal brain lesions from tumor and CVA. They cite nocturnal frequency as the most common manifestation, affecting 36% of their patients. Urgency incontinence occurred in 29%, “voiding difficulty” in 25%, urgency without incontinence in 25%, diurnal frequency in 13%, and enuresis in 6%. Acute retention occurred in only 6%. Fowler (1999) cites studies showing that the presence of urinary incontinence within 7 days of a stroke is a more powerful prognostic indicator for poor survival and functional dependence than a depressed level of consciousness. Urinary incontinence at admission was found by Garibella (2003) to have a hazard ratio of 2.8 as a predictor of stroke death at 3 months. Stroke patients who were incontinent had an increased risk of infectious complications and were malnourished, possible confounders of the increased death risk. Patel and colleagues (2001) reported that urinary incontinence was associated with age older than 75 years, dysphagia, visual field defect, and motor weakness. Certain specific types of strokes also appear to be associated with unusual forms of incontinence. Lenticulo-capsular strokes have been noted to be associated with incontinence. Fifty-two percent of patients with strokes in this area of the brain demonstrated poststroke emotional incontinence, which was not related to other aspects of stroke or gender (Kim, 2002). Lesions centrally located in the globus pallidus, especially dorsally located, were more prone to be associated with an emotional incontinence. This area of the brain is noted to have high density of serotonergic fibers, which has been attributed as an explanation as to why this occurs. Previous descriptions of the voiding dysfunction after CVA have all cited the preponderance of detrusor overactivity with coordinated sphincter activity (Kolominsky-Rabas et al, 2003; Wyndaele et al, 2005). It is difficult to reconcile this with the relatively high incontinence rate that occurs, even considering the probability that a percentage of these patients had an incontinence problem before the CVA. Tsuchida and colleagues (1983) and Khan and colleagues (1990) made early significant contributions in this area by correlating the urodynamic and computed tomographic (CT) pictures after CVA. They reported that patients with lesions in only the basal ganglia or thalamus have normal sphincter function. This means that when an impending involuntary contraction or its onset was sensed, these patients could voluntarily contract the striated sphincter and abort or considerably lessen the effect of an abnormal micturition reflex. The majority of patients with involvement of the cerebral cortex or internal capsule or both were unable to forcefully contract the striated sphincter under these circumstances. Although the authors and others have called this problem “uninhibited relaxation of the sphincter” (Marinkovic and Badlani, 2001), it really is not, but certainly the term does imply that a profound abnormality exists in these patients in the cerebral to corticospinal circuitry that is necessary for voluntary control of the striated sphincter. Griffiths (2004) has summarized the evidence, obtained by electrical stimulation, positron emission tomography (PET), and functional magnetic resonance imaging (fMRI), implicating Barrington nucleus, the so-called M region, as responsible for coordinated striated sphincter relaxation followed by detrusor contraction (i.e., normal voiding). He summarizes evidence that a second region in the pons—the L region—may be responsible for maintaining striated sphincter tone between voids, although the evidence for this is less convincing. Griffiths (1998), studying the results of single-proton emission computer tomography (SPECT) in a group of geriatric patients with established urinary incontinence, found urge incontinence in approximately 50%; and, in half of these there was reduced sensation of bladder filling, more so in men than in women. True urgency incontinence with reduced bladder sensation was associated with global underperfusion of the cerebral cortex, especially the frontal areas, especially on the right. Thus there are two possible mechanisms for the incontinence associated with involuntary bladder contractions in patients who have sustained a CVA: (1) impaired striated sphincter control and (2) lack of appreciation of bladder filling and impending bladder contraction. Smooth sphincter activity is generally unaffected (synergic) by CVA. Some authors describe striated sphincter dyssynergia in 5% to 21% of patients with brain disease and voiding dysfunction (Sakakibara et al, 1999). This is incompatible with accepted neural circuitry. The authors of this chapter agree with those who believe that true detrusor–striated sphincter dyssynergia does not occur in this situation. Pseudodyssynergia may indeed occur during urodynamic testing of these patients (Wein and Barrett, 1982). This refers to an electromyographic (EMG) sphincter “flare” during filling cystometry that is secondary to attempted inhibition of an involuntary bladder contraction by voluntary contraction of the striated sphincter. The guarding reflex is generally intact (Siroky and Krane, 1982). Detrusor hypocontractility or areflexia may persist after CVA. The exact incidence of areflexia as a cause of chronic voiding symptoms after CVA is uncertain. In our patient population, this is rare, but some estimates place it as high as 20% (Arunable and Badlani, 1993). In a group of incontinent male stroke patients, Linsenmeyer and Zorowitz (1992) found 35% had involuntary bladder contractions with urodynamic evidence of bladder outlet obstruction and 6% had detrusor areflexia. In women in this group, 13% had involuntary contraction with a large residual urine volume and 19% had areflexia. Poor flow rates and high residual urine volumes in a male with pre-CVA symptoms of prostatism generally indicate prostatic obstruction, but a full urodynamic evaluation is advisable before committing a patient to mechanical outlet reduction, primarily to exclude detrusor overactivity with impaired contractility as a cause of symptoms. Key Point: Lower Urinary Tract Dysfunction after CVA Other important modifying factors should be considered in the case of these patients. This is generally a problem of the elderly, some of whom have preexistent lower urinary tract pathologic processes. Previously, the problems may have been manageable, but the additional difficulty may make the situation intolerable. As Andrews (1994) notes, other aspects of the brain damage can affect general rehabilitation and control of the lower urinary tract dysfunction. These may include cognitive impairment, dysphasia, inappropriate and aggressive behavior, impaired mobility, and low motivation. Finally, the entire voiding dysfunction may be adversely affected by treatment regimens that concentrate on detrusor overactivity alone (e.g., anticholinergic or antispasmodic therapy). Many such patients are depressed, and confusion and disorientation often result, which compounds the problem. Vigorous pharmacologic therapy of detrusor overactivity with agents that cross the blood-brain barrier and inhibit M1 receptor function may make these associated problems of mentation worse. The underlying basic mechanisms of bladder overactivity after CVA remain unclear. Experimental models of middle cerebral artery occlusion have been described, most recently followed by reperfusion to simulate the clinical condition (Pehrson et al, 2003). Shimizu and colleagues (2003) described the development of a rat model involving an electrolytic lesion of the right basal forebrain. Following at least middle cerebral artery occlusion, the overactivity seems to involve glutaminergic, dopaminergic, and γ-aminobutyric acid (GABA)-ergic innervations (Kanie et al, 2000; Yokoyama et al, 2002). In addition, Fu and colleagues (2004) have shown upregulation of proinflammatory cytokines and neuronal nitric oxide synthetase gene in the spinal cord and bladder. Such findings raise interesting theoretic possibilities for pharmacologic management other than or in addition to antimuscarinic therapy. Sakakibara and colleagues (1996b) studied 39 patients with brainstem stroke. Nineteen of these had voiding symptoms. The major problems were nocturnal frequency and voiding difficulty in six, urinary retention in eight, and urinary incontinence in three. Problems were more common after damage from bleeding than from infarction. Symptoms did not occur in those with strictly midbrain lesions but occurred in 18% of patients with medullary stroke and in 35% of patients with pontine lesions. Eleven patients were symptomatic and underwent urodynamic study. Detrusor overactivity was found in 8 of the 11 and low compliance in 1. What was interpreted as striated sphincter dyssynergia was reported in 5 of the 11 patients, and what was called uninhibited sphincter relaxation occurred in 3. The authors concluded that lesions of the dorsolateral pons involving the pontine reticular nucleus, reticular formation, and the locus coeruleus were mainly responsible for the micturition disturbances in patients with brainstem lesions and that these findings corroborated the presence of a pontine micturition center in humans, corresponding to the pontine storage and micturition centers reported in animal studies. Dementia is a poorly understood disease complex involving atrophy and the loss of gray and white matter of the brain, particularly of the frontal lobes. Problems result with memory and the performance of tasks requiring intellectual mentation. Associated conditions include widespread vascular disease, Alzheimer disease, Pick disease, Jakob-Creutzfeldt disease, syphilis, heat trauma, and encephalitis. Alzheimer disease is the principal cause of dementia in the elderly (Wyndaele et al, 2005, 2009). When voiding dysfunction occurs, the result is generally incontinence. It is difficult to ascertain whether the pathophysiology and considerations are similar to those in the stroke patient or whether the incontinence reflects a situation in which the individual has simply lost the awareness of the desirability of voluntary urinary control. Even if the person has voluntary sphincter control, such individuals may void when and where they please, because mentation fails to dictate why they should not. Such activity may be caused by detrusor overactivity or an otherwise normal, but inappropriately timed, micturition reflex. An accurate estimate of the prevalence of dementia-associated incontinence is confounded by the difficulty in distinguishing this from age-related changes in the bladder and from other concomitant diseases, as pointed out by Wyndaele and colleagues (2005, 2009), who cite figures of 30% to 100%. Treatment is obviously difficult without a desire for improvement. Additionally, therapy that inhibits muscarinic brain receptors may be contraindicated in Alzheimer disease if current theories about its etiology are valid (cortical cholinergic loss). Traumatic brain injury has been cited as the most common form of severe neurologic impairment as a result of trauma (Blaivas and Chancellor, 1995a). When voiding dysfunction occurs, there may be an initial period of detrusor areflexia. With lesions above the pontine micturition center, involuntary bladder contractions are the most frequent manifestation of chronic lower urinary tract dysfunction. Coordinated sphincter function is the rule. In patients who have more isolated brainstem injuries with involvement below the pontine micturition center, detrusor striated sphincter dyssynergia may occur in addition. Chua and colleagues (2003) assessed 66 males and 18 females within 6 weeks of acute traumatic brain injury. Sixty-two percent of patients had urinary incontinence on admission with retention (defined as >100 mL), noted in 9.5%. Sixty-two percent required either indwelling catheters or external collecting devices for urinary maintenance. Urinary incontinence was associated with poor functional status and bilateral lesions, whereas urinary retention was more commonly noted in patients with comorbid diabetes mellitus or fecal impaction. After rehabilitation, 36% remained incontinent (Chua et al, 2003). Both primary and metastatic brain tumors have been reported to be associated with disturbances of bladder function. When dysfunction results, it is related to the localized area involved rather than to the tumor type. The areas that are most frequently involved with associated micturition dysfunction are the superior aspects of the frontal lobe (Blaivas, 1985). When voiding dysfunction occurs, it generally consists of detrusor overactivity and urinary incontinence. These individuals may have a markedly diminished awareness of all lower urinary tract events and, if so, are totally unable to even attempt suppression of the micturition reflex. Smooth and striated sphincter activity is generally synergic. Pseudodyssynergia may occur during urodynamic testing. Fowler (1999) has reviewed the literature on frontal lobe lesions and bladder control. She cites instances of resection of a tumor relieving the micturition symptoms for a period of time, raising the question of whether the phenomenon of tumor-associated bladder overactivity was a positive one (activating some system) rather than a negative one (releasing a system from control). Urinary retention has also been described in patients with space-occupying lesions of the frontal cortex, in the absence of other associated neurologic deficits (Lang et al, 1996). Posterior fossa tumors may be associated with voiding dysfunction (32% to 70%, based on references cited by Fowler, 1999). Retention or difficulty voiding is the rule, with incontinence being rarely reported. This group of diseases involves pathologic degeneration of the nervous system generally involving the cerebellum, but with a possible extension to the brainstem, spinal cord, and dorsal nerve roots (Leach et al, 1982). Poor coordination, depressed deep tendon reflexes, dysarthria, dysmetria, and choreiform movements result because of the cerebellar involvement. Voiding dysfunction is generally manifested by incontinence, usually associated with detrusor overactivity and sphincter synergia. Retention or high residual urine volume may occur as well. Poor emptying, when present, is most commonly caused by detrusor areflexia, but it may be associated with detrusor–striated sphincter dyssynergia, presumably secondary to spinal cord involvement. Sakakibara and colleagues (1998b) reported micturitional symptoms in 184 patients with spinocerebellar degeneration. Twenty-nine (15.8%) showed stress urinary incontinence. Although 20 of these 29 also had detrusor overactivity and/or low compliance and/or residual urine, 9 had none of these findings. The authors speculate that in some such patients, spinal lesions affecting Onuf nucleus and consequently pudendal nerve function were responsible. This is a disease of progressive dementia and ataxia occurring in patients with normal cerebrospinal fluid pressure and distended cerebral ventricles but with no passage of air over the cerebral convexities by pneumoencephalography (Blaivas, 1985). When voiding dysfunction occurs, it is generally incontinence secondary to detrusor overactivity with sphincter synergia. Cerebral palsy (CP) is the name applied to a nonprogressive injury of the brain in the prenatal or perinatal or postnatal period (generally during the first year of life, but some say up to 3 years) that produces neuromuscular disability and/or specific symptom complexes of cerebral dysfunction. The etiology is generally infection or a period of hypoxia. Affected children exhibit delayed gross motor development, abnormal motor performance, altered muscle tone, abnormal posture, and exaggerated reflexes. Most children and adults with only CP have urinary control and what seems to be normal filling/storage and normal emptying. The actual incidence of voiding dysfunction is somewhat vague because the few available series report findings predominantly in those who present with voiding symptoms. Andrews (1994) estimates that a third or more of children with CP are so affected. Roijen and colleagues (2001) surveyed children and adolescents from six rehabilitation centers and cited the prevalence of “primary urinary incontinence” as 23.5%. The most important factors influencing the occurrence of incontinence were spastic tetraplegia and low intellectual capacity. Wyndaele and colleagues (2005, 2009) cite the occurrence of lower urinary tract dysfunction as 36%. When an adult with CP presents with an acute or subacute change in voiding status, however, it is most likely unrelated to CP. Reid and Borzyskowski (1993) described findings in 27 patients, aged 3 to 20 years, referred for voiding dysfunction. Incontinence (74%), frequency (56%), and urgency (37%) were the most common presenting symptoms, and detrusor overactivity was the most common urodynamic abnormality (87% of those undergoing urodynamics), with 25% of these exhibiting apparent striated sphincter dyssynergia. Mayo (1992) reported on 33 CP patients referred for evaluation, of whom 10 were older than 20 years. Difficulty urinating was the predominant symptom in about half the patients, but half of these also had overactivity and urgency when the bladder was full. The cause of the difficulty in voluntarily initiating micturition was thought to be a problem with relaxing the pelvic floor and not true striated sphincter dyssynergia. Incontinence was the major presenting symptom in the other half, associated in 14 of 16 with detrusor overactivity; all exhibited normal voiding otherwise. Decreased sensation was reported in 17 of 23 patients younger than 20 years of age and in 4 of 10 older than 20. The more serious manifestations such as retention were found only in the adults, prompting the author to suggest that difficulty urinating may progress in adulthood. Reid and Borzyskowski (1993) note that incontinence can be significantly improved in most CP patients and that, in their experience, intellectual delay is not a barrier to successful management. However, one special problem that is encountered in some of these individuals that makes their management difficult is a severe degree of mental retardation, such that any evaluation or treatment that requires cooperation is virtually impossible. With such individuals, sometimes the best that one can do is to check the upper tracts with renal ultrasonography, measure serum creatinine levels, and obtain an estimate of postvoid residual urine, either by catheterization or ultrasound, and proceed accordingly. In those individuals with CP who exhibit significant dysfunction, the type of damage that one would suspect from the most common urodynamic abnormalities seems to be localized anatomically above the brainstem. This is commonly reflected by phasic detrusor overactivity and coordinated sphincters. However, spinal cord damage can occur, and perhaps this accounts for those individuals with CP who seem to have evidence of striated sphincter dyssynergia or of a more distal type of neural axis lesion. Parkinson disease (PD) is a neurodegenerative disorder of unknown cause that affects primarily the dopaminergic neurons of the substantia nigra but also heterogeneous populations of neurons elsewhere (Lang and Lozano, 1998). The most important site of pathology is the substantia nigra pars compacta, the origin of the dopaminergic nigrostriatal tract to the caudate nucleus and putamen. Dopamine deficiency in the nigrostriatal pathway accounts for most of the classic clinical motor features of PD. The classic major signs of PD consist of tremor, skeletal rigidity, and bradykinesia, a symptom complex often referred to as parkinsonism. Positron-emission tomography (PET) has shown alteration in brain activation in response to bladder filling in PD. PET revealed changes in nine patients in brain activation associated with detrusor overactivity, specifically in the periaqueductal gray, the supplementary motor area, the cerebellar vermis, the insula, putamen, and thalamus. The most prominent degree of increased activation was noted in the cerebellum, with no change in pons during detrusor overactivity (Kitta et al, 2006). The role of alterations in dopaminergic receptor subtypes has been assessed in animal models of PD. D-2 agonists and D-1 antagonists appear to result in a reduction of bladder capacity in these models. Brusa and colleagues (2006) studied a group of 87 patients with mild PD who were evaluated by symptomatic change and urodynamics after administration of selective dopaminergic agents. Agents resulting in acute central D-2 stimulation result in bladder capacity and worsened detrusor overactivity, as compared with peripheral dopaminergic antagonists (Brusa et al, 2006). There are causes of parkinsonism other than PD, and in an excellent review, Lang and Lozano (1998) discuss clinical distinguishing features of these other causes of parkinsonism from PD. These other causes consist of (1) multiple system atrophy (MSA: includes striatonigral degeneration, sporadic olivopontocerebellar atrophy, and Shy-Drager syndrome); (2) progressive supranuclear palsy; (3) cortical-basal ganglionic degeneration; (4) so-called vascular parkinsonism; and (5) dementia with Lewy bodies. The combination of asymmetry of symptoms and signs, the presence of a resting tremor, and a good response to levodopa best differentiates PD from parkinsonism produced by other causes, although none of these is individually specific for PD. Wyndaele and colleagues (2005) endorse additional criteria for distinguishing lower urinary tract symptoms caused by MSA from those caused by PD. The following suggest MSA: (1) urinary symptoms precede or present with parkinsonism; (2) urinary incontinence; (3) significant postvoid residual; (4) initial erectile failure; and (5) abnormal striated sphincter electromyogram. Five percent of patients initially diagnosed with PD are found to have Parkinson’s Plus Syndromes. These syndromes are characterized by early dementia and/or falls, symmetric symptoms such as a wide-based gait, normal eye movements, autonomic dysfunction, and marked disability. These variants tend to have a worse prognosis than does idiopathic PD. Urinary function is not well described in this subgroup of patients (Nutt and Wooten, 2005). The “gold standard” for the diagnosis of PD is the neuropathologic examination. In addition to the characteristic pattern of the loss of selected populations of neurons, there is the presence of degenerating ubiquitin-positive neuronal processes or neurites (Lewy neurites) found in all affected brainstem regions. The Lewy body is an intracytoplasmic eosinophilic hyaline inclusion consistently observed in selectively vulnerable neuronal populations, although these are not specific to PD, being found in small numbers in other neurodegenerative disorders. PD affects both sexes roughly equally. The prevalence is cited as 0.3% of the general population and 3% of people older than 65 years (Lang and Lozano, 1998). Voiding dysfunction occurs in 35% to 70% of patients with PD (Berger et al, 1990; Sotolongo and Chancellor, 1993; Blaivas et al, 1998a; Wein and Rovner, 1999; Wyndaele et al, 2005, 2009). Preexisting detrusor or outlet abnormalities may be present, and the symptomatology may be affected by various types of treatment for the primary disease. When voiding dysfunction occurs, symptoms generally (50% to 75%) consist of urgency, frequency, nocturia, and urge incontinence. The remainder of patients have “obstructive” symptoms or a combination. The most common urodynamic finding is detrusor overactivity. The pathophysiology of detrusor overactivity most widely proposed (Fowler, 1999) is that the basal ganglia normally have an inhibitory effect on the micturition reflex, which is abolished by the cell loss in the substantia nigra. Whether the dopamine D1 or D2 receptor (or both) are primarily responsible does not seem to have been settled as yet. It has been suggested that loss of inhibitory D1-like receptors causes detrusor overactivity, allowing D2 receptors to facilitate micturition (Andersson and Wein, 2004). The smooth sphincter is synergic. There is some confusion regarding EMG interpretation. Sporadic involuntary activity in the striated sphincter during involuntary bladder contraction has been reported in up to 60% of patients; however, this does not cause obstruction and cannot be termed true dyssynergia, which generally does not occur. Pseudodyssynergia may occur, as well as a delay in striated sphincter relaxation (bradykinesia) at the onset of voluntary micturition, both of which can be urodynamically misinterpreted as true dyssynergia. Impaired detrusor contractility may also occur, either in the form of low amplitude or poorly sustained contractions or a combination. Detrusor areflexia is relatively uncommon in PD. It should be recognized, however, that many cases of “PD” in the older literature may actually have been MSA, and citations regarding symptoms and urodynamic findings may not therefore be accurate. A good and important example of this is the inference from the publication by Staskin and colleagues (1988) that transurethral prostatectomy (TURP) in the patient with PD is associated with a high incidence of urinary incontinence (because of poor striated sphincter control). Retrospective interpretation (Fowler, 1999, 2001; Wyndaele et al, 2005) has shown that these were patients with MSA and not PD and that TURP should not be contraindicated in patients with PD because external sphincter acontractility is extremely rare in such patients. However, one must be cautious with such patients, and a complete urodynamic or video-urodynamic evaluation is advisable. Poorly sustained bladder contractions, sometimes with slow sphincter relaxation, should make one less optimistic regarding the results of outlet reduction in the male. PD has been shown to impact voiding dysfunction in postradical prostatectomy patients. Routh and colleagues (2006) noted that in a study of retrospective evaluation of 20 patients with PD who had undergone radical prostatectomy that de novo incontinence rate was 24% and was of a minimal nature on the basis of pad utilization. However, the conclusion of the study was that advanced evaluation preoperatively including urodynamic or video-urodynamic evaluation would seem advisable for purposes of counseling given the higher rates of incontinence in this particular population. Christmas and colleagues (1988) demonstrated that subcutaneous administration of a dopamine receptor agonist (apomorphine) can reliably and rapidly reverse parkinsonian “off” periods (periods of worsening symptoms mainly caused by the timing of previous medication doses and the unpredictable nature of motor fluctuations). By repeating video-urodynamic studies during the motor improvement after administration of apomorphine, bladder outlet obstruction secondary to benign prostatic hyperplasia (BPH) may be able to be distinguished from voiding dysfunction secondary to PD. The authors also point out that apomorphine might be useful in such patients who have severe off-phase voiding dysfunction (e.g., those with disabling nocturnal frequency and incontinence). Voiding dysfunction secondary to PD defies “routine” classification within any system. It is most manifest by storage failure secondary to bladder overactivity, but detailed urodynamic evaluation is mandatory before any but the simplest and most reversible therapy. The therapeutic menus (see Tables 66–3 and 66–4) are perfectly applicable, but the disease itself may impose certain limitations on the use of certain treatments (e.g., limited mobility for rapid toilet access, hand control insufficient for clean intermittent catheterization [CIC]). Animal models of PD have been developed, using injections of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine or 6-hydroxydopamine into the nigrostriatal pathway (Yoshimura et al, 2003; Andersson and Wein, 2004; Wyndaele et al, 2005). Subthalamic nucleus deep brain stimulation has been shown to be effective for motor symptoms and dyskinesias in patients with moderate to severe PD. Clinical studies have shown that this type of stimulation improves urinary function in these patients by ameliorating bladder sensation and also improving functional bladder capacity. Herzog and colleagues (2006) studied 11 patients undergoing deep brain stimulation with positron emission tomography assessments of regional cerebral blood flow. Subjects were studied with urodynamics both with stimulation on and stimulation off. At urodynamic capacity, significant increases in anterior cingulate regional blood flow were noted. They were increased when deep brain stimulation was off. At bladder capacity, there was also an increase in lateral frontal cortical blood flow with stimulation off. These results were felt to be indicative of deep brain stimulation improvement of bladder function by the modulation of afferent bladder information to cortical and subcortical areas (Herzog et al, 2006). Multiple system atrophy is a progressive neurodegenerative disease of unknown etiology. The symptoms encompass parkinsonism and cerebellar, autonomic (including urinary and erectile problems), and pyramidal cortical dysfunction in a multitude of combinations. The clinical features and the differentiation from PD are nicely described in a consensus statement by Gilman and colleagues (1999). These investigators advocate a designation of MSA-P if parkinsonian features predominate and one of MSA-C if cerebellar features predominate. Older names such as “striatonigral degeneration,” “sporadic olivopontocerebellar atrophy,” and “Shy-Drager syndrome” (Wein, 2002a) should be discarded in favor of these. The neurologic lesions of MSA consist of cell loss and gliosis in widespread areas, much more so than with PD, and this more diffuse nature of cell loss probably explains why bladder symptoms may occur earlier than in PD and be more severe and why erection may be affected as well (Kirby et al, 1986; Beck et al, 1994; Chandiramani et al, 1997). Affected areas have been identified in the cerebellum, substantia nigra, globus pallidus, caudate, putamen, inferior olives, intermediolateral columns of the spinal cord, and Onuf nucleus. Males and females are equally affected, with the onset in middle age. MSA is generally progressive and associated with a poor prognosis. Shy-Drager syndrome has been described in the past as characterized clinically by orthostatic hypotension, anhidrosis, and varying degrees of cerebellar and parkinsonian dysfunction. Voiding and erectile dysfunction are common. Some consider this as late-stage MSA (Chandiramani et al, 1997). Chandiramani and colleagues (1997) compared the clinical features of 52 patients with probable MSA and 41 patients with PD. Of patients with MSA, 60% had their urinary symptoms precede or present with their symptoms of parkinsonism. Of patients with PD, 94% had been diagnosed for several years before the onset of urinary symptoms. In patients with MSA, urinary incontinence was a significant complaint in 73%, whereas 19% had only frequency and urgency without incontinence. Sixty-six percent of the patients with MSA had a significant postvoid residual volume (100 to 450 mL). In patients with PD, frequency and urgency were the predominant symptoms in 85%, whereas incontinence was the primary complaint in 15%. In only 5 of 32 patients with PD in whom residual urine volume was measured was it significant. Eleven men with MSA underwent TURP. Nine of these had deterioration of their urinary incontinence afterward. All three women with MSA were incontinent after pelvic floor repair. Five men with PD underwent prostatectomy, and three reported a good result. Ninety-three percent of the men with MSA questioned about erectile function reported erectile failure, and in 13 of 27 of these the erectile dysfunction preceded the diagnosis of MSA. Seven of the 21 men with PD had erectile failure, but in all these men the diagnosis of erectile dysfunction followed the diagnosis of PD by 1 to 4 years. Fowler (2001) lists the clinical urogenital criteria favoring a diagnosis of MSA as (1) urinary symptoms precede or present with parkinsonism; (2) male erectile dysfunction precedes or presents with parkinsonism; (3) urinary incontinence; (4) significant postvoid residual; and (5) worsening lower urinary tract dysfunction after urologic surgery. The initial urinary symptoms of MSA are urgency, frequency, and urgency incontinence, occurring up to 4 years before the diagnosis is made, as does erectile failure. Detrusor overactivity is frequently found, as one would expect from the central nervous system areas affected, but decreased compliance may occur, reflecting distal spinal involvement of the locations of the cell bodies of autonomic neurons innervating the lower urinary tract. As the disease progresses, difficulty in initiating and maintaining voiding may occur, probably from pontine and sacral cord lesions, and this is generally associated with a poor prognosis. Cystourethrography or video-urodynamic studies may reveal an open bladder neck (intrinsic sphincter deficiency), and many patients exhibit evidence of striated sphincter denervation on motor unit electromyography. The smooth and striated sphincter abnormalities predispose women to sphincteric incontinence and make prostatectomy hazardous in men. Berger and coworkers (1990) described a useful urodynamic differentiation of what was termed Shy-Drager syndrome (probable late stage MSA) from PD. Parkinsonian patients with voiding dysfunction generally have detrusor overactivity and normal compliance. An open bladder neck was seen only in patients with “Shy-Drager syndrome,” excluding those patients with PD who have had a prostatectomy. EMG evidence of striated sphincter denervation was seen much more commonly in those diagnosed as having Shy-Drager syndrome. Multiple sclerosis (MS) is primarily a disease of young and middle-aged adults with a twofold predilection for women. Litwiller and colleagues (1999) detailed current prevalence rates for MS as 1/1000 Americans, 2/1000 northern Europeans, and 20 to 40/100,000 first-degree relatives of patients with MS. The disease is believed to be immune mediated and is characterized by neural demyelination, generally characterized by axonal sparing, in the brain and spinal cord (Noseworthy et al, 2000). This demyelination causes impairment of saltatory conduction and of conduction velocity in axonal pathways, resulting in various neurologic abnormalities that are subject to exacerbation and remission. Lesions, known as plaques, range from 1 mm to 4 cm and are scattered throughout the white matter of the nervous system (Chancellor and Blaivas, 1993). The demyelinating process most commonly involves the lateral corticospinal (pyramidal) and reticulospinal columns of the cervical spinal cord, and it is thus not surprising that voiding dysfunction and sphincter dysfunction are so common. Autopsy studies have revealed almost constant evidence of demyelination in the cervical spinal cord, but involvement of the lumbar and sacral cord occurs in approximately 40% and 18%, respectively (Blaivas and Kaplan, 1988). Lesions may also occur in the optic nerve and in the cerebral cortex and midbrain, the latter accounting for the intellectual deterioration and/or euphoria that may be seen as well (Kirby, 1994; Noseworthy et al, 2000), ultimately in up to 43% to 65% of patients (Litwiller et al, 1999). A rat model for a demyelinating disease resembling MS has been described using myelin basic protein as an antigen for inducing experimental allergic encephalomyelitis (Mizusawa et al, 2000). The incidence of voiding dysfunction in MS is related to the disability status. Of patients with MS, 50% to 90% complain of voiding symptoms at some time; the prevalence of incontinence is cited as 37% to 72% (Wyndaele et al, 2005). Litwiller and colleagues (1999), in their excellent review article, cite symptoms of frequency or urgency in 31% to 85% of patients in various series, with corresponding percentages of incontinence as 37% to 72% and of obstructive symptoms with urinary retention in 2% to 52%. Lower urinary tract involvement may constitute the sole initial complaint or be part of the presenting symptom complex in up to 15% of patients, usually in the form of acute urinary retention of “unknown” etiology or as an acute onset of urgency and frequency, secondary to overactivity (Wyndaele et al, 2005). Detrusor overactivity is the most common urodynamic abnormality detected, occurring in 34% to 99% of cases in reported series (Blaivas and Kaplan, 1988; Chancellor and Blaivas, 1993; Sirls et al, 1994; Litwiller et al, 1999). Of the patients with overactivity, 30% to 65% have coexistent striated sphincter dyssynergia. Impaired detrusor contractility or areflexia may also exist; estimates of prevalence range from 12% to 38% (Wyndaele et al, 2005), a phenomenon that can considerably complicate treatment efforts. Other estimates suggest that 62% of patients with MS have neurogenic detrusor overactivity with bladder outlet obstruction, 25% have neurogenic detrusor overactivity with detrusor sphincter dyssynergia, 20% have detrusor under activity, and 10% have no initial abnormal urodynamic findings. The variability and potential multiplicity of lesions associated with MS, however, prohibit accurate diagnosis on the basis of urodynamics alone (Ukkonen et al, 2004). Bladder dysfunction associated with MS, as compared with women with non-neurogenic detrusor activity, has been shown to demonstrate significant differences. The amplitude of the first overactive contraction is greater in patients with MS as compared with those patients with idiopathic detrusor overactivity, as is the maximal detrusor contraction. The threshold volume at which detrusor overactivity occurs in patients with neurogenic detrusor overactivity, however, is somewhat higher than in those patients with idiopathic detrusor overactivity, which may be related to residual volume and which is noted in patients with detrusor overactivity. Using threshold values for prediction of type of voiding dysfunction, 30 cm of H2O and above was predictive of patients having neurogenic detrusor activity as compared with those who have non-neurogenic detrusor overactivity. These differences in neurogenic detrusor overactivity urodynamic findings may be representative of direct neurogenic influences on the detrusor muscle from abnormal bladder interpolation and/or signaling as compared with non-neurogenic patients (Lemack, 2006). Generally, the smooth sphincter is synergic. Chancellor and Blaivas (1993) reviewed urodynamic findings in multiple series of patients with MS and voiding dysfunction and summarized the incidence of three basic patterns: (1) detrusor overactivity, striated sphincter synergia 26% to 50% (average 38%); (2) detrusor overactivity, striated sphincter dyssynergia 24% to 46% (average, 29%); and (3) detrusor areflexia 19% to 40% (average, 26%). Litwiller and colleagues (1999) tabularized 22 references, reporting approximately the same ranges. It is also possible to see relative degrees of sphincteric flaccidity caused by MS, a phenomenon cited as occurring in less than 15% of patients (Litwiller et al, 1999); this finding could predispose and contribute to sphincteric incontinence. De Ridder and colleagues (1998) reported weakness of pelvic floor contraction in nearly all 30 women they studied with MS. Spasticity of the pelvic floor was present in all patients with striated sphincter dyssynergia but in none with detrusor overactivity alone. Because sensation is frequently intact in these patients, one must be careful to distinguish urodynamic pseudodyssynergia from true striated sphincter dyssynergia. Blaivas and colleagues (1981) subcategorized true striated sphincter dyssynergia in patients with MS and identified some varieties that are more worrisome than others. For instance, in a female with MS, a brief period of striated sphincter dyssynergia during detrusor contraction but one that does not result in excessive intravesical pressure during voiding, substantial residual urine volume, or secondary detrusor hypertrophy may be relatively inconsequential, whereas those varieties that are more sustained—resulting in high bladder pressures of long duration—are most associated with urologic complications. Giannantoni and colleagues (1998) reviewed 116 of their patients and likewise concluded that there was a significant relationship between the maximum amplitude of the involuntary bladder contractions and upper urinary tract deterioration. Chancellor and Blaivas (1993) emphasized what they believed were the most important parameters predisposing patients with MS to significant urologic complications: (1) striated sphincter dyssynergia in men; (2) high detrusor filling pressure (>40 cm H2O); and (3) an indwelling catheter. Interestingly, however, Wyndaele’s committee (Wyndaele et al, 2005) concluded that progressive neurologic disease, MS included, rarely causes upper urinary tract damage, even when, in MS, severe spasticity and disability exist. The reason for this is unknown, but they believe that the situation and concerns with respect to this are unlike those for SCI. Key Point: Multiple Sclerosis Aggressive and anticipatory medical management can obviate most significant complications. Sirls and colleagues (1994) reported that only 7% (the author calculated 10.4%) of their patients required surgical intervention because of failure of aggressive medical management and that none developed hydronephrosis on such therapy. The regimens used were (1) drugs to decrease detrusor overactivity plus CIC (57%); (2) such drugs alone (13%); (3) CIC alone (15%); and (4) behavioral therapy. A significant proportion of patients with MS with and without new symptoms will develop changes in their detrusor compliance and urodynamic pattern (Ciancio et al, 2001). Caution should therefore be exercised in recommending irreversible therapeutic options. No factors appear to be predictive of upper tract changes in MS. In a 4-year follow-up of multiple sclerosis patients, 113 patients were evaluated, of whom 66 patients had both urodynamic and renal ultrasound testing. Sixteen percent (11 patients) had abnormal ultrasound findings. The most significant finding was minor caliectasis, which was of no clinical significance. Neither creatinine nor urodynamic findings were associated with the abnormal renal ultrasound findings (Lemack et al, 2005). Surgical intervention for multiple sclerosis appears to be diminishing with improved pharmacologic management and the realization of the alternating neurologic picture of lower urinary dysfunction associated with MS (Ukkonen et al, 2004). No consensus on optimal bladder management has identified for multiple sclerosis, and most commonly, management is predicated on symptomatic and urodynamic findings. According to consensus agreement, De Ridder and colleagues (2005) concluded that in early multiple sclerosis, anticholinergics and intermittent catheterization were considered to be cornerstones of therapy. For advanced MS (with an EDSS > 7), specific guidelines remain lacking. In general, crédé voiding or Valsalva voiding are contraindicated, especially in the presence of detrusor sphincter dyssynergia. They further recommended that indwelling catheters be reserved for patients for whom all other possible treatments have failed. In the approximately 30% of patients with MS using indwelling urinary catheters, the suprapubic route is the preferred route in both men and women. This form of management is considered reasonable for that subpopulation, as long as long-term follow-up is continued (De Ridder et al, 2005). SCI may occur as a consequence of acts of violence, fracture, or dislocation of the spinal column secondary to motor vehicle, diving accidents or falls, vascular injuries or repair, infection, disk prolapse, or sudden or severe hyperextension from other causes. Altered lower urinary tract and sexual function frequently occur secondary to SCI and significantly affect quality of life; SCI patients are at risk urologically for urinary tract infection, sepsis, upper and lower urinary tract deterioration, upper and lower urinary tract calculi, autonomic hyperreflexia (dysreflexia), skin complications, and depression (which can complicate their urologic management). Failure to properly address the lower urinary tract dysfunction can lead to significant morbidity and mortality. There is great variation in urologic practice regarding initial evaluation, follow-up, and surveillance among spinal injury units (Bycroft et al, 2004), a problem that Boone (2004) properly attributes to a lack of evidence-based decision making. Stover and Fine (1987) reviewed the epidemiology and other general aspects of SCI. The annual rate was reported as 30 to 32 new SCIs per million persons at risk in the United States; the prevalence was approximately 906 per million. This coincides roughly with the estimate by DeVivo (1997) of approximately 10,000 new cases of SCI in the United States yearly and an estimate of 12,000 per year by Rabchevsky and Smith (2001). DeVivo (1997) reported the most common mechanisms of injury, as collected by the National Spinal Cord Injury Statistical Center, as motor vehicle accidents (35.9%), violence (29.5%), falls (20.3%), and sports-related injuries (7.3%). Males account for 71% to 80% of patients with SCI. The average age at injury is 31.5 years. Children comprise 3% to 5% of all patients with SCI (Generao et al, 2004). Stover and Fine (1987) reported that neurologically incomplete quadriplegics constituted the largest group of SCI patients at the time of hospital admission (28%), followed by complete paraplegics (26%), complete quadriplegics (24%), and incomplete paraplegics (18%). This seems to have changed little during the 1990s. The majority occur at or above the T12 spinal column (vertebral) level. Although earlier data (Hackler, 1977) indicated that renal disease was the major cause of death, at least in the paraplegic patient, a retrospective study of more than 5000 patients who sustained SCI between 1973 and 1980 revealed that the leading causes of death at that time were pneumonia, septicemia, heart disease, accidents, and suicide (Stover and Fine, 1987; Soden et al, 2000). These figures seemingly indicate a distinct improvement in the urologic care of these patients. Impaired mobility is commonly noted in the spinal cord–injured patient and may substantially affect urinary habit and incontinence (Biering-Sorensen et al, 2004). Urologic phenomena also figure prominently in chronic SCI. Within 10 years after SCI, approximately 7% of patients with spinal cord will develop an initial kidney stone with the greatest risk occurring during the first 3 months after injury. Ninety-eight percent of these stones will be apatite or struvite in composition. There appear to be two time frames for stone formation in this population, one being the acute phase associated with immobilization and immobilization hypercalciuria. The other is a more chronic phase stone formation period, usually associated with chronic catheter management years after injury and predominately involving the lower tract (Post and Noreau, 2005). Controlled and coordinated lower urinary tract function depends on an intact neural axis. Bladder contractility and the occurrence of reflex contractions depend on an intact sacral spinal cord and its afferent and efferent connections (see Chapter 61). Key Point: Spinal Cord Injury An impressive amount of literature is continuously building on the neurobiology of the spinal cord and its acute and chronic alteration after SCI. These topics are not specifically considered in detail here, nor are the ramifications of this information relative to potential improvement of spinal cord function after injury by stem cell implant or reinnervation. Earlier reviews can be found by Olson (1997), Fawcett (1998), and Kakulas (1999); later ones were presented by Rabchevsky and Smith (2001) (this also includes a discussion of pathophysiology and experimental models), Cao and colleagues (2002) (stem cell repair), Fawcett (2002) (repair of SCI), Rossi and Cattaneo (2002) (stem cell therapy), Mitsui and colleagues (2003) (stem cell repair), Kakulas (2004) (neuropathology and natural history of the spinal cord changes), and Livshits and colleagues (2004) (reinnervation). Sexual and reproductive dysfunction in the patient with SCI is a topic that deserves much attention in the overall rehabilitation plan. Pertinent general and specific concepts of sexual and reproductive dysfunction and their normalization in this special group of patients can be found in Chapters 23, 27 (male), and 30 (female). Other excellent reviews on the specifics of sexual function in SCI can be found by Bennett and colleagues (1988), Stone and MacDermott (1989), Smith and Bodner (1993), and Biering-Sorensen and Sonksen (2001), and on infertility by Linsenmeyer and Perkash (1991), Rajaskaran and Monga (1999), and Rutkowski and colleagues (1999). After a significant SCI, a period of decreased excitability of spinal cord segments at and below the level of the lesion occurs, referred to as “spinal shock.” There is absent somatic reflex activity and flaccid muscle paralysis below this level. Although classic teaching refers to generalized areflexia below the level of the lesion for days to months, Thomas and O’Flynn (1994) confirm that the most peripheral somatic reflexes of the sacral cord segments (the anal and bulbocavernosus reflexes) may never disappear or, if they do, may return within minutes or hours of the injury. However, functions proximal to the level of the injury may be depressed as well (Atkinson and Atkinson, 1996). Although the course of spinal shock is well known, the actual phenomenon remains poorly understood, with little or no basic research evident recently. Spinal shock includes a suppression of autonomic activity, as well as somatic activity, and the bladder is acontractile and areflexic. Radiologically, the bladder has a smooth contour with no evidence of trabeculation. The bladder neck is generally closed and competent unless there has been prior surgery or in some cases of thoracolumbar and presumably sympathetic injury (Sullivan and Yalla, 1992). The smooth sphincter mechanism seems to be functional. Some EMG activity may be recorded from the striated sphincter, and the maximum urethral closure pressure is lower than normal but still maintained at the level of the external sphincter zone; however, the normal guarding reflex (striated sphincter response during filling) is absent and there is no voluntary control (Fam and Yalla, 1988
Objectives
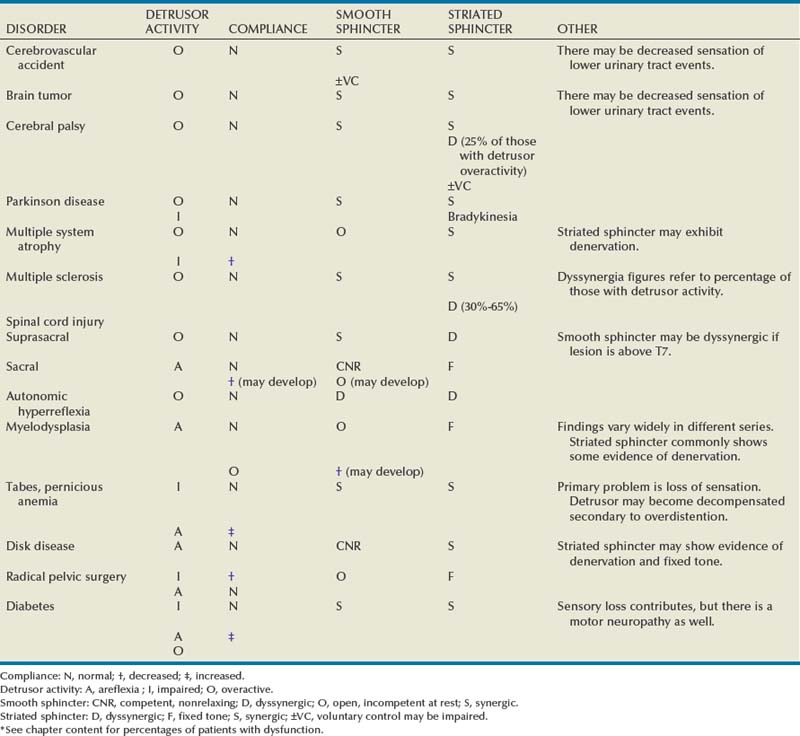
General Patterns of Neuropathic Lower Urinary Tract Dysfunction
Plasticity
Disease at or Above the Brainstem
Cerebrovascular Disease
Cerebrovascular Accident (Stroke)
Brainstem Stroke
Dementia
Traumatic Brain Injury
Brain Tumor
Cerebellar Ataxia
Normal-Pressure Hydrocephalus
Cerebral Palsy
Parkinson Disease
Multiple System Atrophy
Diseases Primarily Involving the Spinal Cord
Multiple Sclerosis
Spinal Cord Injury
Epidemiology, Morbidity, General Concepts
Spinal Shock
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neuromuscular Dysfunction of the Lower Urinary Tract
• Neurologic lesions above the brainstem (with rare exceptions) that affect micturition generally result in involuntary bladder contractions with coordinated sphincter function (smooth and striated sphincter synergy). Sensation and voluntary striated sphincter function are generally preserved, but sensation may be deficient or delayed. Detrusor areflexia may, however, occur, either initially or as a permanent dysfunction. Urinary incontinence may occur due to the detrusor overactivity.
• Patients with complete lesions of the spinal cord between spinal cord level T6 and S2, after they recover from spinal shock, generally exhibit involuntary bladder contractions without sensation, smooth sphincter synergy, but striated sphincter dyssynergia. Those with lesions above spinal cord level T6 may experience, in addition, smooth sphincter dyssynergia and autonomic hyperreflexia (see later). Incontinence may occur due to detrusor overactivity, but the outlet obstruction can also cause urinary retention and overflow incontinence.
• Patients with significant spinal cord or nerve root trauma or disease below spinal cord level S2 generally do not manifest involuntary bladder contractions per se. Detrusor areflexia is initially the rule after spinal shock, and, depending on the type and extent of neurologic injury, various forms of decreased compliance during filling (usually due to bladder wall fibrosis) may occur. An open smooth sphincter area may result, but whether this is caused by sympathetic or parasympathetic decentralization/defunctionalization (or both or neither) has never been determined. Various types of striated sphincter dysfunction may occur, but commonly the area retains a residual resting sphincter tone (not the same as dyssynergia) and is not under voluntary control.
• The dysfunctions that occur with interruption of the peripheral reflex arc may be similar to those of distal spinal cord or nerve root injury. Detrusor areflexia often develops, low compliance may result, the smooth sphincter area may be relatively incompetent, and the striated sphincter area may exhibit fixed residual tone not amenable to voluntary relaxation. True peripheral neuropathy can be motor or sensory, with the usual expected sequelae, at least initially.
• In the functional system of classification (see Chapter 66), the most common type of voiding dysfunction after stroke would then be characterized as a failure to store secondary to bladder overactivity, specifically involuntary bladder contractions. In the ICS Classification System, the dysfunction would most likely be classified as overactive neurogenic detrusor function, normal sensation, low capacity, normal compliance, and normal urethral closure function during storage; during voiding the description would be normal detrusor activity and normal urethral function assuming that no anatomic obstruction existed. Treatment, in the absence of coexisting significant bladder obstruction or significantly impaired contractility, is directed at decreasing bladder contractility and increasing bladder capacity (see Tables 61–3 and 61–4).
• The most common functional classification applicable to patients with voiding dysfunction secondary to MS would thus be storage failure secondary to detrusor overactivity. This is commonly complicated by striated sphincter dyssynergia, with varying effects on the ability to empty completely at acceptable pressures. Other abnormalities and other combined deficits, are obviously possible, however. Once the dysfunction is broadly characterized, the treatment options should be obvious from the therapeutic menus (see Tables 61–3 and 61–4).
• Generally, complete spinal cord lesions above the sacral spinal cord, but below the area of the sympathetic outflow, result in detrusor overactivity, absent sensation below the level of the lesion, smooth sphincter synergia, and striated sphincter dyssynergia. Lesions at or above the spinal cord level of T7 or T8 (the spinal column level of T6) may result in smooth sphincter dyssynergia as well. However, although the correlation between neurologic and urodynamic findings is good, it is not perfect, and a neurologic examination is no substitute for a urodynamic evaluation in these patients when one is determining risk factors and treatment (see later).





