Andrew J. Stephenson, MD, FACS, FRCSC, Timothy D. Gilligan, MD, MS Neoplasms of the testis comprise a morphologically and clinically diverse group of tumors, 95% of which are germ cell tumors (GCTs). GCTs are broadly categorized as seminoma and nonseminoma (NSGCT) owing to differences in natural history and treatment. GCTs are relatively rare malignancies, accounting for 1% to 2% of cancers among men in the United States, with an incidence of approximately 5 cases per 100,000. Approximately 90% of GCTs arise in the testis and 2% to 5% are extragonadal (the retroperitoneum and mediastinum are the most common sites). With the development of cisplatin-based chemotherapy and the integration of surgery, GCTs have become a model of a curable neoplasm and serve as a paradigm for the multidisciplinary treatment of cancer (Einhorn, 1981). Before therapy with cisplatin was introduced the cure rate for patients with advanced GCT was 5% to 10%. Currently the long-term survival for men with metastatic GCT is 80% to 90%. With the successful cure of patients (even those with advanced disease), an important treatment objective is minimizing treatment-related toxicity without compromising curability. Mortality from GCT is due to inherent resistance to platin chemotherapy and the failure to fully eradicate residual disease elements in the early course of therapy. Non-GCTs of the testis are rare and include sex cord/stromal tumors, lymphoid and hematopoietic tumors, tumors of the collecting duct and rete testis, and tumors of the testicular adnexa. A classification of neoplasms of the testis is provided in Table 31–1. Table 31–1 World Health Organization Classification of Testicular Tumors
| Germ Cell Tumors |
| Sex Cord/Gonadal Stromal Tumors |
| Unclassified Forms |
| Tumors Containing Both Germ Cell and Sex Cord/Gonadal Stromal Elements |
| Miscellaneous Tumors |
| Lymphoid and Hematopoietic Tumors |
| Tumors of Collecting Ducts and Rete |
| Tumors of the Tunica, Epididymis, Spermatic Cord, Supporting Structures, and Appendices |
| Soft Tissue Tumors |
| Unclassified Tumors |
| Secondary Tumors |
| Tumor-like Lesions |
Data from Vogelzang NJ, Scardino PT, Shipley WU, Coffey DS, editors. Genitourinary oncology. Philadelphia: Lippincott Williams & Wilkins; 1999.
Germ Cell Tumors
Epidemiology
As of 2010 in the United States it was estimated that 8400 men would develop testicular cancer and 380 would die of this disease (Jemnal et al, 2010). In the United States, testicular cancer is the most common malignancy among men aged 20 to 40 years and the second most common cancer after leukemia among males aged 15 to 19 years (Horner et al, 2009). Testicular tumors have three age model peaks: infancy, ages 30 to 34 years, and approximately age 60. The incidence of bilateral GCT is approximately 2% (Fossa et al, 2005). Testicular lymphoma is less common than GCT but accounts for the majority of testicular tumors in men older than 50 years of age and is more likely to have a synchronous bilateral presentation.
The incidence of testicular cancer varies significantly according to geographic region: rates are highest in Scandinavia, Germany, Switzerland, and New Zealand; intermediate in the United States and Great Britain; and lowest in Africa and Asia. The incidence of testicular cancer in the United States in non-Hispanic whites is five times higher than the incidence in African-Americans, four times higher than the incidence in Asians, and 78% higher than in Hispanics (Horner et al, 2009).
The incidence of GCT appears to be increasing worldwide (McKiernan et al, 1999; McGlynn et al, 2005; Purdue et al, 2005). In the United States, the age-adjusted incidence rate for males aged 15 to 49 years increased from 2.9 per 100,000 in 1975 to 5.1 per 100,000 in 2004 (Holmes et al, 2008). Over this time period incidence rates have increased substantially more for seminoma than NSGCT (McGlynn et al, 2005; Powles et al, 2005). A stage migration of GCT has been observed in several countries owing, in part, to increased awareness and earlier diagnosis. Between 1973 and 2001 the percentage of tumors diagnosed at a localized stage increased from 55% to 73% in the United States among white males. The stage distribution for African-American males remained stable during this time (McGlynn et al, 2005). Only 10% to 30% of men will present with distant metastatic disease. In the United Kingdom the change in stage distribution over time is largely restricted to an increase in localized seminoma and a decrease in metastatic NSGCT; rates of localized NSGCT and metastatic seminoma are largely unchanged (McGlynn et al, 2005). Currently, localized seminoma is the most common presentation of GCT, representing approximately 50% of all men with GCT (Powles et al, 2005). Thus, contemporary testicular germ cell tumors have more favorable prognostic features on average compared with those diagnosed in the 1970s and 1980s.
Risk Factors
There are four well-established risk factors for testicular cancer: cryptorchidism, family history of testicular cancer, a personal history of testicular cancer, and intratubular germ cell neoplasia (ITGCN). Infertile men also have a higher incidence of testicular cancer. Numerous studies have reported that recent increases in the incidence of testicular cancer can be largely attributed to birth-cohort effects, which implies that diet and/or other environmental factors play a major role in GCT carcinogenesis (Liu et al, 1999; Huyghe et al, 2003; McGlynn et al, 2003; Richiardi et al, 2004; Bray et al, 2006; Verhoeven et al, 2008).
Males with cryptorchidism are four to six times more likely to be diagnosed with testicular cancer, but the relative risk (RR) falls to 2.0 to 3.0 if orchidopexy is performed before puberty (Dieckmann and Pichlmeier, 2004; Wood and Elder, 2009). Although most of the increased risk reflects an increased risk of cancer in the undescended testis, a meta-analysis of cryptorchidism studies reported that the contralateral descended testis is also at slightly increased risk (RR 1.74 [95% CI, 1.01 to 2.98]) (Akre et al, 2009). Men with a first-degree relative with testicular cancer have a substantially increased risk, and the median age at diagnosis in these men is 2 to 3 years younger than in the general population (Mai et al, 2009). An individual’s RR for testicular cancer is 8.0 to 12.0 with an affected brother compared with 2.0 to 4.0 in those with an affected father (Westergaard et al, 1996; Sonneveld et al, 1999; Hemminki and Chen, 2006). Men with a history of testicular cancer are at a 12-fold increased risk of developing GCT in the contralateral testis, but the 15-year cumulative incidence is only 2%. The risk is higher in patients who are younger when testicular cancer is diagnosed and in men whose initial GCT is seminoma (Theodore et al, 2004; Fossa et al, 2005). A population-based study of contralateral testicular cancer reported that a man younger than the age of 30 with a testicular seminoma has a 3.1% risk of developing a contralateral testicular cancer (Fossa et al, 2005). The same study reported that 10-year overall survival after diagnosis with a second primary (i.e., contralateral) testicular cancer was 93%.
Most GCTs arise from a precursor lesion called intratubular germ cell neoplasia (ITGCN) (which is also referred to as carcinoma in situ). Men with ITGCN have a significantly increased risk of developing invasive GCT. ITGCN is present in adjacent testicular parenchyma in 80% to 90% cases of invasive GCT and is associated with a 50% risk of GCT within 5 years and a 70% risk within 7 years (Skakkebaek et al, 1982; Dieckmann and Skakkebaek, 1999; Montironi, 2002). Between 5% and 9% of patients with GCT have ITGCN within the unaffected contralateral testis, although the incidence of contralateral ITGCN increases to about 36% in men with testicular atrophy or cryptorchidism (Dieckmann and Loy, 1996; Dieckmann and Skakkebaek, 1999). Gene expression profile analysis indicates that ITGCN develops before birth from an arrested gonocyte (Hussain et al, 2008; Sonne et al, 2009). In men with a history of GCT the finding of testicular microlithiasis on ultrasound evaluation of the contralateral testis is associated with an increased risk of ITGCN (Karellas et al, 2007). However, the significance of microlithiasis in the general population is unclear; a study of 1500 army volunteers found a 5.6% prevalence of microlithiasis, yet fewer than 2% of those with microlithiasis developed cancer within the subsequent 5 years (DeCastro et al, 2008).
Pathogenesis and Biology
The carcinogenesis of GCTs is poorly understood. As noted earlier, testicular GCTs develop from a precursor lesion, ITGCN, which, in turn, appears to develop from arrested primordial germ cells or gonocytes that failed to differentiate into prespermatogonia (Rajpert-de Meyts and Hoei-Hansen, 2007; Hussain et al, 2008). These cells are thought to then lie dormant until after puberty when they are stimulated by increased testosterone levels.
The increased incidence of testicular cancer that started in the first half of the 20th century has been accompanied by an increased incidence of other male reproductive disorders, such as hypospadias, cryptorchidism, and subfertility (Rajpert-de Meyts and Hoei-Hansen, 2007; Sonne et al, 2008). These findings led to the hypothesis that testicular cancer and these other disorders all resulted from a testicular dysgenesis syndrome, which in turn resulted from environmental and/or lifestyle factors and genetic susceptibility. The specific environmental or lifestyle factors have not been defined. Increased prenatal estrogen exposure has been hypothesized as a risk factor, but this is controversial (Martin et al, 2008). There is stronger evidence that reduction in androgen activity can result in features of testicular dysgenesis syndrome, including cryptorchidism, hypospadias, and impaired spermatogenesis, but a direct link between reduced androgen signaling and ITGCN or GCTs remains hypothetical (Sonne et al, 2008; Hu et al, 2009).
Evidence of environmental and lifestyle factors contributing to testicular cancer includes the rapid rise in its incidence as well as findings that the risk in second-generation immigrants is similar to that in their country of birth. In addition, mothers of children with testicular cancer (but not the testicular cancer patients themselves) have been found to have higher blood levels of certain organic pollutants compared with other mothers (Sonne et al, 2008). Evidence for genetic factors includes the clustering of testicular cancer in some families, the extreme difference in the rate of testicular cancer in black and white Americans, and the finding of susceptibility loci on chromosomes 5, 6, and 12 in case-control studies (Mai et al, 2009). In addition, specific polymorphisms of certain genes, including the gene encoding c-KIT ligand, have been associated with an increased risk of testicular cancer (Blomberg Jensen et al, 2008; Kanetsky et al, 2009). Gonocytes depend on c-KIT ligand for survival, and the gene for this protein is located on the short arm of chromosome 12. Approximately 70% of GCTs have an extra copy of chromosome 12 in the form of an isochromosome 12p (i[12p]) (Bosl et al, 1989). Thus a connection between mutations or polymorphisms in this gene and GCT has biologic plausibility.
One of the most striking features of GCTs is their sensitivity to cisplatin-based chemotherapy, which enables cure in the vast majority of patients with widely metastatic disease. The specific biologic basis of this acute vulnerability to chemotherapy remains incompletely understood but is thought to derive from the close relationship between GCTs and embryonal stem cells and gonocytes, which have a low threshold for undergoing apoptosis in response to DNA damage (Mayer et al, 2003; Schmelz et al, 2010). GCTs have high intrinsic levels of wild-type TP53 protein (which plays a role in mediating cell cycle arrest and apoptosis), and TP53 mutations in GCTs are rare, yet differences have not been consistently found in TP53 status when comparing chemosensitive and chemoresistant germ cell tumors (Burger et al, 1998; Houldsworth et al, 1998). Similarly, expression of the antiapoptotic protein BCL2 is low in germ cell tumors but BCL2 levels do not distinguish chemosensitive and chemoresistant cell lines (Mayer et al, 2003). Gene expression analysis has found an upregulation of numerous genes that facilitate apoptosis, including FASLG, TNFSF10, and BAX, whereas BCL2 is downregulated (Schmelz et al, 2010). Expression patterns of genes controlling the G1/S-phase checkpoint in GCTs appear to promote induction of apoptosis (Schmelz et al, 2010). In addition, GCTs lack transporters to export cisplatin from the cell and have a reduced ability to repair cisplatin-induced DNA damage (Mayer et al, 2003). Nonetheless, a small fraction of GCTs are resistant to chemotherapy and the basis of that resistance remains obscure, although DNA mismatch repair deficiency, microsatellite instability, and BRAF mutations have been associated with treatment failure (Honecker et al, 2009).
Up to 10% of GCTs are extragonadal and develop in midline anatomic locations. There are two main competing theories regarding the pathogenesis of extragonadal GCTs. The first hypothesizes that they originate from germ cells that mismigrated along the genital ridge and were able to survive in an extragonadal environment. The second theory proposes a reverse migration from the testis to extragonadal locations (Chaganti and Houldsworth, 2000).
Primary mediastinal NSGCTs differ in several ways from those originating in the testis or retroperitoneum. First, they are less sensitive to chemotherapy and have a poor prognosis with a 5-year overall survival of about 45% (Bokemeyer et al, 2002b). Mediastinal NSGCTs are more likely to have yolk sac tumor components and thus to be associated with elevations in serum α-fetoprotein (AFP) (Kesler et al, 2008). They are also associated with Kleinfelter syndrome and with hematologic malignancies that carry extra copies of the short arm of chromosome 12, as seen in adult GCTs (Bokemeyer et al, 2002a; McKenney et al, 2007). In contrast, mediastinal seminomas carry a similar prognosis to testicular seminomas. Primary retroperitoneal GCTs are indistinguishable biologically from testicular GCTs and carry the same prognosis.
Histologic Classification
The histologic classification of GCT is outlined in Table 31–2 (Sobin and Wittekind, 2002). GCTs are broadly classified as seminoma and NSGCT, and the relative distribution of each is 52% to 56% and 44% to 48%, respectively (McGlynn et al, 2005; Powles et al, 2005). NSGCTs include embryonal carcinoma (EC), yolk sac tumor, teratoma, and choriocarcinoma subtypes, either alone as pure forms or in combination as mixed GCT with or without seminoma (Ulbright, 2005). Most NSGCTs are mixed tumors that are composed of two or more GCT subtypes. GCTs that contain both NSGCT subtypes and seminoma are classified as NSGCT.
Table 31–2 World Health Organization Classification of Germ Cell Tumors
From Sobin LH, Wittekind CH. UICC: TNM classification of malignant tumors. 6th ed. New York: Wiley-Liss; 2002.
Intratubular Germ Cell Neoplasia
With the exception of spermatocytic seminoma, all adult invasive GCTs arise from ITGCN. ITGCN consists of undifferentiated germ cells that have the appearance of seminoma that are located basally within the seminiferous tubules. The tubule usually shows decreased or absent spermatogenesis, and normal constituents are replaced by ITGCN. The presence of ITGCN in an orchiectomy specimen in men with testicular cancer does not carry any prognostic implications with regard to the risk of relapse of the cancer (von Eyben et al, 2004). ITGCN is much less frequent in pediatric GCTs (Cheville, 1999).
Seminoma
Seminoma is the most common type of GCT. On average, seminomas occur at an older average age than NSGCTs, with most cases diagnosed in the fourth or fifth decade of life (Rayson et al, 1998). Grossly, seminoma is a soft tan to white diffuse or multinodular mass (Fig. 31–1A). Necrosis may be present but is usually focal and not as prominent as other GCTs. Seminomas consist of a sheetlike arrangement of cells with polygonal nuclei and clear cytoplasm, with the cells divided into nests by fibrovascular septa that contain lymphocytes (see Fig. 31–1B) (Ulbright, 2005). Syncytiotrophoblasts, which stain positive for human choriogonadotropin (hCG), can be identified in about 15% of cases of pure seminoma but are of no clear prognostic significance (Cheville, 1999). Lymphocytic infiltrates and granulomatous reactions are often seen and seminomas appear to be associated with an increased incidence of sarcoidosis (Rayson et al, 1998; Tjan-Heijnen et al, 1998). Seminomas may be confused with solid-pattern EC, yolk sac tumor, or Sertoli cell tumors (Ulbright and Young, 2008). Although immunohistochemical staining plays a limited role in diagnosing GCTs, seminomas are typically negative for CD30, positive for CD117, and strongly positive for placental alkaline phosphatase (PLAP). Anaplastic seminoma was a previously recognized subtype of seminoma, but this distinction is of no clear biologic or clinical significance and is no longer recognized. Seminoma arises from ITGCN and is considered to be the common precursor for the other NSGCT subtypes (Ulbright, 2004). This ability of seminoma to transform into NSGCT elements has important therapeutic implications for the management of seminoma (discussed later) (Ulbright, 2004).
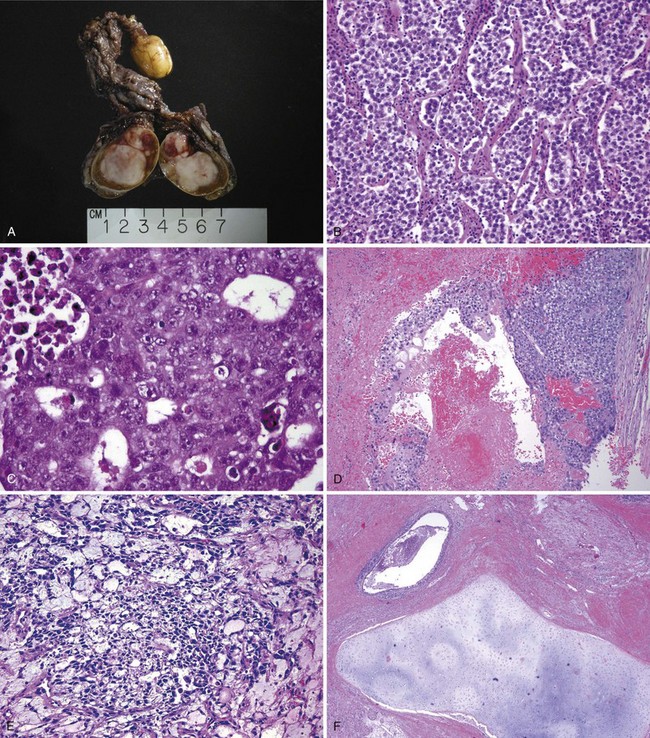
(A-F, Courtesy of Donna E. Hansel, MD, PhD.)
Spermatocytic Seminoma
Spermatocytic seminoma is rare and accounts for less than 1% of GCTs. Although classified as a variant of seminoma it represents a distinct clinicopathologic entity from other GCTs. Unlike other GCTs, spermatocytic seminoma does not arise from ITGCN, is not associated with a history of cryptorchidism or bilaterality, does not express PLAP or i(12p), and does not occur as part of mixed GCTs (Ulbright, 2005). Histopathologically, it differs from seminoma in that the nuclei are round, minimal lymphocytic infiltration is present, and three distinct cell types are present, including small lymphocyte-like cells, medium-sized cells with dense eosinophilic cytoplasm and a round nucleus, and large mononucleated or multinucleated cells (Aggarwal and Parwani, 2009). The peak incidence is in the sixth decade of life (Eble, 1994; Chung et al, 2004a). It is a benign tumor (only one documented case having metastasized) and is almost always cured with orchiectomy (Chung et al, 2004a). An exception to this is the rare case of spermatocytic seminoma with sarcoma that exhibits elements of sarcomatous differentiation and is commonly associated with widely metastatic disease and poor prognosis.
Embryonal Carcinoma
EC consists of undifferentiated malignant cells resembling primitive epithelial cells from early-stage embryos with crowded pleomorphic nuclei (Ulbright, 2005). Grossly, EC is a tan to yellow neoplasm that often exhibits large areas of hemorrhage and necrosis. The microscopic appearance of these tumors varies considerably, and they may grow in solid sheets or in papillary, glandular-alveolar, or tubular patterns (see Fig. 31–1C). In some cases, syncytiotrophoblasts are identified. EC is an aggressive tumor associated with a high rate of metastasis, often in the context of normal serum tumor markers. EC is the most undifferentiated cell type of NSGCT, with totipotential capacity to differentiate to other NSGCT cell types (including teratoma) within the primary tumor or at metastatic sites. As discussed later, the presence and proportion of EC has been associated with an increased risk of occult metastases in clinical stage (CS) I NSGCT. EC typically stains for AE1/AE3, PLAP, and OCT3/4 and does not stain for c-KIT.
Choriocarcinoma
Choriocarcinoma is a rare and aggressive tumor that typically presents as elevated serum hCG levels and disseminated disease. Choriocarcinoma commonly spreads by hematogenous routes, and common sites of metastases include lungs and brain, but eye and skin metastases have also been reported (Tinkle et al, 2001; Osada et al, 2004). Microscopically the tumor is composed of syncytiotrophoblasts and cytotrophoblasts, and the former stain positively for hCG (see Fig. 31–1D) (Cheville, 1999). Seminoma and EC may also contain syncytiotrophoblasts. Areas of hemorrhage and necrosis are prominent. As in gestational trophoblastic disease, testicular choriocarcinoma is prone to hemorrhage, sometimes both spontaneously and immediately after chemotherapy is initiated, and such bleeding can be catastrophic, particularly when it occurs in the lungs or brain (Motzer et al, 1987).
Yolk Sac Tumor
Pure yolk sac tumors (sometimes called endodermal sinus tumors) represent a very small fraction of adult-type GCTs but are more common in mediastinal and pediatric GCTs. Mixed GCTs often include elements of yolk sac tumor, which consists of a reticular network of medium-sized cuboidal cells with cytoplasmic and extracytoplasmic eosinophilic, hyaline-like globules (Epstein, 2010). Hyaline globules are a characteristic feature and are present in up to 84% of cases. Yolk sac tumors may grow in a glandular, papillary, or microcystic pattern. A characteristic feature is the Schiller-Duval body, which resembles endodermal sinuses, and is seen in roughly half of cases (see Fig. 31–1E). Yolk sac tumors almost always produce AFP but not hCG. Among men with CS I NSGCT with normal serum tumor markers the presence of a yolk sac tumor is associated with a lower risk of relapse, but this finding may simply be a result of serum tumor markers (i.e., AFP) having a higher sensitivity for detecting micrometastatic disease in this type of GCT (Read et al, 1992).
Teratoma
Teratomas are tumors that contain well-differentiated or incompletely differentiated elements of at least two of the three germ cell layers of endoderm, mesoderm, and ectoderm. Characteristically all components are intermixed. Well-differentiated tumors are labeled mature teratomas, whereas those that are incompletely differentiated (i.e., similar to fetal or embryonal tissue) are called immature teratomas. Mature teratomas may include elements of mature bone, cartilage, teeth, hair, and squamous epithelium, a fact that most likely explains the name teratoma, which roughly means “monster tumor,” in Greek (see Fig. 31–1F). The gross appearance of teratoma depends largely on the elements within it, with most tumors having solid and cystic areas. Teratomas are generally associated with normal serum tumor markers, but they may cause mildly elevated serum AFP levels. Approximately 47% of adult mixed GCTs contain teratoma, but pure teratomas are uncommon. In contrast, pure teratomas comprise a sizeable fraction of pediatric GCTs (Epstein, 2010).
Despite their benign histologic appearance, teratomas may contain genetic abnormalities frequently found in malignant GCT elements, including aneuploidy, i(12p), and widely variable proliferative capacity (Castedo et al, 1989; Sella et al, 1991). Studies have also shown that cystic fluid from teratoma frequently contains hCG and AFP, confirming the malignant potential of teratoma (Sella et al, 1991; Beck et al, 2004). The genetic instability of teratoma has important clinical implications. Teratomas may grow uncontrollably, invade surrounding structures, and become unresectable (known as growing teratoma syndrome) (Logothetis et al, 1982). On rare occasions, teratoma may transform into a somatic malignancy such as rhabdomyosarcoma, adenocarcinoma, or primitive neuroectodermal tumor (Little et al, 1994; Comiter et al, 1998; Motzer et al, 1998). These tumors are called “teratoma with somatic-type malignancy” or “teratoma with malignant transformation.” They frequently have abnormalities of chromosome 12 or i(12p), indicating their origin from GCT. Malignant transformation is highly aggressive, resistant to conventional chemotherapy, and associated with a poor prognosis. Lastly, unresected teratoma in patients with advanced NSGCT may result in late relapse (Sheinfeld, 2003). All of these events may have lethal consequences.
Initial Presentation
Diagnostic Delay
Although testicular cancer is the most common malignancy in young adult males, diagnostic delay is a well-recognized phenomenon of this disease, with patients and physicians contributing to this delay. Testicular cancer patients are typically young and may be less inclined to seek medical evaluation for symptoms due to denial, ignorance, or limited access. In cases of diagnostic delay, the longest period of delay has been attributed to the physician in up to two thirds of cases. Prior studies show that up to one third of testicular tumors are initially misdiagnosed as epididymitis or hydrocele (Bosl et al, 1981). For patients who present with signs or symptoms from metastatic GCT, these may become the focus of the treating physician, resulting in the failure to diagnose GCT. These patients may be subjected to inappropriate treatment, diagnostic tests, and unnecessary surgery with subsequent delays in definitive therapy. Case reports describe patients undergoing exploratory laparotomy, neck dissection, or mastectomy for unsuspected metastatic GCT. The interval of delay is associated with advanced clinical stage, suboptimal response to chemotherapy, and diminished survival. Moul and colleagues (1990) reported a decrease in survival in GCT patients treated from 1970 to 1987 with a diagnostic delay greater than 16 weeks, although a significant survival difference was not observed among patients treated in the cisplatin era. Stephenson and associates (2004) reported a higher proportion of men requiring intensive chemotherapy (multiple regimens, high-dose, and salvage chemotherapy) among those with a treatment delay greater than 30 days due to unnecessary exploratory laparotomy.
Diagnostic Testing and Initial Management
Scrotal Ultrasonography
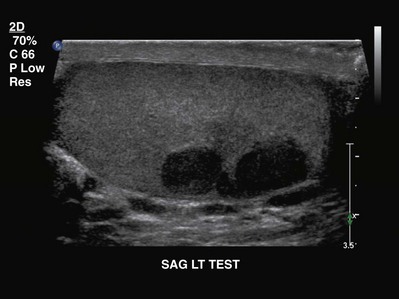
In men presenting with a testicular mass, hydrocele, or unexplained scrotal symptoms or signs, scrotal ultrasonography should be considered an extension of the physical examination because it is widely available, inexpensive, and noninvasive. With high-frequency transducers (5 to 10 MHz), intratesticular lesions as small as a few millimeters can be identified and readily distinguished from an extratesticular pathologic process. On ultrasonography the typical GCT is hypoechoic and two or more discrete lesions may be identified (Fig. 31–2). Heterogeneous echotexture within a lesion is more commonly associated with NSGCT, because seminomas usually have a homogeneous echotexture. The presence of increased flow within the lesion on color Doppler ultrasonography is suggestive of malignancy, although its absence does not exclude GCT. The association between testicular microlithiasis and GCT is not clearly defined, and this finding alone should not prompt further evaluation (DeCastro et al, 2008). Given the 2% incidence of bilateral GCTs, both testes should be evaluated ultrasonographically, although bilateral tumors at diagnosis is rare (0.5% of all GCTs) and metachronous presentation is more common (Fossa et al, 2005).
The presence of small (<10 mm), impalpable intratesticular lesions in the absence of disseminated GCT or elevated serum tumor markers represents a diagnostic dilemma. The majority of these lesions are benign (testicular cysts, small infarcts, Leydig cell nodules, or small Leydig cell or Sertoli cell tumors), although up to 20% to 40% may represent small GCTs (usually seminoma) (Hindley et al, 2003; Connolly et al, 2006; Muller et al, 2006). Management options include inguinal orchiectomy, inguinal exploration and excision (with frozen section analysis to rule out GCT), and close observation with serial ultrasonographic evaluation (with exploration of growing lesions). Intraoperative ultrasonography is useful during surgical exploration of the testis to locate the lesion. The risk of malignancy increases with the size of the lesion (Carmignani et al, 2005).
Serum Tumor Markers
Patients suspected of having a GCT should have blood drawn for serum AFP, hCG, and LDH evaluation before orchiectomy to aid in the diagnosis and to help interpret postorchiectomy tumor marker levels. For staging purposes it is relevant to know whether preorchiectomy serum tumor marker levels are declining after orchiectomy and, if so, how quickly. The results of serum tumor marker assays should not be used to guide decision making about whether to perform a radical orchiectomy, because AFP or hCG levels in the normal range do not rule out GCT. A significantly elevated serum AFP level can establish the diagnosis of NSGCT in a patient whose histopathologic diagnosis is pure seminoma because seminomas do not produce AFP. However, borderline-elevated values should be interpreted cautiously. In rare patients who present with a testicular, retroperitoneal, or mediastinal primary tumor and whose disease burden has resulted in a need to start treatment very urgently, substantially elevated serum AFP and/or hCG levels may be considered sufficient for diagnosis of GCT. For rare patients with medically unstable disease, treatment need not be delayed until histology results permit a tissue diagnosis. However, these patients should undergo radical orchiectomy after the completion of chemotherapy because the testis is a sanctuary site for malignant GCT owing to the blood-testis barrier and because the testis frequently contains residual invasive GCT, teratoma, and/or ITGCN (Geldart et al, 2002).
Radical Inguinal Orchiectomy
Histopathologic examination of the testis should identify the histologic type of the tumor (see Table 31–2) (Sobin and Wittekind, 2002), tumor size, multifocality, local tumor invasion (rete testis, tunica albuginea, tunica vaginalis, epididymis, spermatic cord, scrotum), primary T stage (Table 31–3) (Sobin and Wittekind, 2002; Greene et al, 2002), presence of ITGCN, invasion of blood or lymphatic vessels (termed lymphovascular invasion [LVI]), and the surgical margin status. For patients with mixed GCT, each individual tumor subtype should be identified, including its relative proportion. Because of the relative rarity of GCT and the importance of primary tumor histology for treatment decision making, review of primary tumor specimens by experienced pathologists is recommended (Krege et al, 2008a). In a recent randomized, multicenter clinical trial, 5 of 382 NSGCT specimens (1.3%) were reclassified as seminomas by centralized pathologic review (Albers et al, 2008).
Testis-Sparing Surgery
Testis-sparing surgery (or partial orchiectomy) is highly controversial and has no role in the patient suspected of having a testicular neoplasm with a normal contralateral testis. However, it may be considered for organ-confined tumors of less than 2 cm in patients with synchronous bilateral tumors or tumor in a solitary testis with sufficient testicular androgen production. It may also be considered for suspected benign tumor or indeterminate lesion when serum AFP, hCG, and LDH values are normal. Testis-sparing surgery is seldom feasible for larger tumors (>2 cm) because a complete excision frequently leaves insufficient residual testicular parenchyma for preservation. When testis-sparing surgery is performed, biopsies of the adjacent testicular parenchyma should be done to rule out the presence of ITGCN. ITGCN is present in adjacent testicular parenchyma in 80% to 90% cases of GCT and is associated with a 50% risk of GCT within 5 years and 70% within 7 years (Skakkebaek et al, 1982; Dieckmann and Skakkebaek, 1999; Montironi, 2002). Adjuvant radiotherapy to the residual testis using doses of 20 Gy or greater is usually sufficient to prevent the development of a GCT. The German Testicular Cancer Study Group reported no cases of local recurrence over a median follow-up of 91 months in 46 patients with small, organ-confined tumors who underwent testis-sparing surgery and received adjuvant radiotherapy for ITGCN (Heidenreich et al, 2001).
Biopsy of the Contralateral Testis
Between 5% and 9% of patients with GCT have ITGCN in the normal contralateral testis (Dieckmann and Skakkebaek, 1999). In patients with an atrophic testis, history of cryptorchidism, or age younger than 40 years, the risk of ITGCN in the contralateral testis has been reported in up to 36% (Dieckmann and Loy, 1996). Thus an open inguinal biopsy of the contralateral testis may be considered in patients with risk factors for ITGCN or those with suspicious lesions on preoperative ultrasonography (Motzer et al, 2006).
Suspected Extragonadal GCT
Two to 5 percent of GCT are extragonadal origin (Bokemeyer et al, 2002b). Of the patients with metastatic GCT without a testicular mass, only one third definitively has a primary extragonadal GCT, because one third has ITGCN in the testis and one third has ultrasonographic evidence of a “burned-out” primary tumor (Scholz et al, 2002). GCT should be considered in any young male with a midline mass. The presence of elevated serum AFP and/or hCG with a normal testicular evaluation is sufficient for the diagnosis of GCT, and histologic confirmation by biopsy is not necessary before starting treatment. When serum tumor markers are normal, then biopsy of the mass should be performed to confirm the diagnosis of GCT before beginning treatment. A biopsy specimen showing poorly differentiated carcinoma represents a diagnostic dilemma if a primary tumor site cannot be confirmed. In this scenario the diagnosis of extragonadal GCT with malignant transformation may be considered and supported by the expression of i(12p) in biopsy specimens.
Clinical Staging
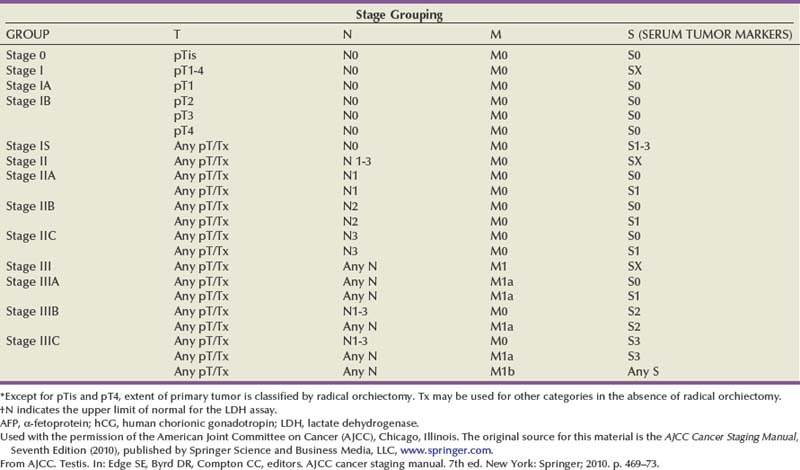
The prognosis of GCT and initial management decisions are dictated by the clinical stage of the disease, which is based on the histopathologic findings and pathologic stage of the primary tumor, postorchiectomy serum tumor marker levels, and presence and extent of metastatic disease as determined by physical examination and staging imaging studies. In 1997 an international consensus classification for GCT was developed by the American Joint Committee on Cancer (AJCC) and Union Internationale Contre le Cancer (UICC) (see Table 31–3). The AJCC and UICC staging systems for GCT are unique because, for the first time, a serum tumor marker category (S) based on postorchiectomy AFP, hCG, and LDH levels is used to supplement the prognostic stages as defined by anatomic extent of disease. The AJCC and UICC staging systems were updated in 2002 and the new systems consider the presence of LVI in the primary tumor as pT2 in an otherwise organ-confined tumor. CS I is defined as disease clinically confined to the testis, CS II indicates the presence of regional (retroperitoneal) lymph node metastasis, and CS III represents nonregional lymph node and/or visceral metastasis.
Staging Imaging Studies
GCT follows a predictable pattern of metastatic spread that has contributed to its successful management. With the exception of choriocarcinoma, the most common route of disease dissemination is via lymphatic channels from the primary tumor to the retroperitoneal lymph nodes and subsequently to distant sites. Choriocarcinoma has a propensity for hematogenous dissemination. The retroperitoneum is the initial site of metastatic spread in 70% to 80% of patients with GCT. Detailed mapping studies from retroperitoneal lymph node dissection (RPLND) series have increased our understanding of the testicular lymphatic drainage and identified the most likely sites of metastatic spread (Sheinfeld, 1994). For right testicular tumors the primary drainage site is the interaortocaval lymph nodes inferior to the renal vessels, followed by the paracaval and para-aortic nodes. The primary “landing zone” for left testicular tumors is the para-aortic lymph nodes, followed by the interaortocaval nodes (Donohue et al, 1982). The pattern of lymph drainage in the retroperitoneum is from right to left. Thus, contralateral spread from the primary “landing zone” is common with right-sided tumors but is rarely seen with left-sided tumors and usually is associated with bulky disease. More caudal deposits of metastatic disease usually reflect retrograde spread to distal iliac and inguinal lymph nodes secondary to large volume disease and, more rarely, aberrant testicular lymphatic drainage. Retroperitoneal lymphatics drain into the cisterna chyli behind the right renal artery and right crus of the diaphragm. Thus, retrocrural lymph node metastasis may be visible in patients with retroperitoneal disease. From there, lymphatic spread occurs via the thoracic duct to the posterior mediastinum and left supraclavicular fossa.
Clinical Staging of the Abdomen and Pelvis
Enlarged retroperitoneal lymph nodes are found on CT in 10% to 20% of seminomas and 60% to 70% of NSGCT. The retroperitoneum continues to be the most difficult area to accurately stage clinically. A consistent 25% to 35% rate of pathologically involved retroperitoneal lymph nodes has been reported for CS I NSGCT in the presence of a “normal” CT scan despite the improvements in CT over the past 4 decades (Fernandez et al, 1994). There is no consensus regarding size criteria for retroperitoneal lymph nodes that constitutes a “normal” CT scan. A size cutoff of 10 mm is frequently used to identify enlarged lymph nodes, but false-negative rates up to 63% have been reported when this size criterion is used. Among patients with CS IIA and IIB disease, clinical overstaging by CT (i.e., pathologically negative lymph nodes at RPLND despite enlarged lymph nodes on CT) is reported in 12% to 40% of patients.
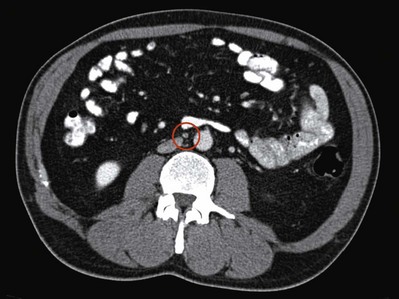
An understanding of the primary drainage sites for left- and right-sided tumors has led to efforts to increase the sensitivity of abdominopelvic CT by decreasing the size criteria for clinically positive lymph nodes in the primary “landing zone,” and a size criterion as small as 4 mm has been proposed. Leibovitch and colleagues (1995) showed that using a size cutoff of 4 mm in the primary landing zone and 10 mm outside this region was associated with a sensitivity and specificity for pathologic stage II disease of 91% and 50%, respectively. In a similar study, Hilton and associates (1997) reported a sensitivity and specificity of 93% and 58%, respectively, using a cutoff of 4 mm for lymph nodes in the primary “landing zone” that were anterior to a horizontal line bisecting the aorta. Based on this evidence, retroperitoneal lymph nodes greater than 5 to 9 mm in the primary “landing zone,” particularly if they are anterior to the great vessels on transaxial CT images, should be viewed with suspicion for regional lymph node metastasis (Fig. 31–3). Because of the rapid growth of GCT, it is advisable to base the management decision on CT studies performed within 4 weeks of the initiation of treatment.
Malignant GCT accumulates fluorodeoxyglucose (FDG), and several studies have investigated FDG-labeled positron emission tomography (FDG-PET) in the staging of GCT at diagnosis and assessing response after chemotherapy. Several small pilot studies suggested that FDG-PET can identify retroperitoneal metastasis in low-stage seminoma and NSGCT more precisely than CT (Albers et al, 1999). In a prospective trial of centrally reviewed FDG-PET studies in 111 contemporary patients with CS I NSGCT on surveillance, relapse was observed in 33 of 87 patients who were PET negative, with an estimated relapse-free rate of 63% (Huddart et al, 2007). The investigators concluded that FDG-PET is not sufficiently sensitive to accurately stage CS I NSGCT. De Wit and associates (2008) also reported that FDG-PET yielded only slightly better results than CT as a primary staging tool for low-stage NSGCT. Thus there is currently no role for FDG-PET in the routine evaluation of NSGCT and seminoma at the time of diagnosis.
Pathologic Staging of the Abdomen and Pelvis
In select centers European centers performing open RPLND and most laparoscopic RPLND series, RPLND is performed in patients with CS I or IIA NSGCT largely as a staging procedure without curative intent to identify the presence of regional lymph nodes and determine the need for subsequent chemotherapy (Nelson et al, 1999; Janetschek et al, 2000; Albers et al, 2003, 2008; Bhayani et al, 2003; Nielsen et al, 2007). Pathologic N stage differs from clinical N stage in that the former also considers the number of lymph nodes involved:
Chest Imaging
All patients with GCT should undergo chest imaging before decisions regarding management are made. Thoracic metastasis in the absence of retroperitoneal disease and/or elevated serum tumor markers is uncommon, particularly for seminoma. Thus, routine chest CT may be associated with a high rate of false-positive findings, which may complicate subsequent therapy (Horan et al, 2007). Thus it is reasonable to perform obtain chest radiographs at the time of diagnosis as an initial staging study, and CT should be performed in patients with elevated postorchiectomy levels of serum tumor markers, evidence of metastatic disease by physical examination or abdominopelvic CT, or abnormal or equivocal findings on chest radiography. It may be reasonable to perform chest CT in patients with CS I NSGCT with evidence of LVI or EC predominance because some studies have reported a high rate of hematogenous metastasis to the lung in the setting of a negative CT for retroperitoneal metastasis (Hermans et al, 2000; Sweeney et al, 2000). Mediastinal or hilar lymphadenopathy in the absence of retroperitoneal disease should raise the index of suspicion of non-GCT etiology such as lymphoma or sarcoidosis, and histologic confirmation of GCT by mediastinoscopy and biopsy should be performed before initiating systemic therapy (Hunt et al, 2009).
Prognostic Classification of Advanced GCT
An international, retrospective pooled analysis of 5202 patients with advanced NSGCT treated between 1975 and 1990 with platin-containing chemotherapy regimens (cisplatin or carboplatin) identified AFP, hCG, and LDH levels at the initiation of chemotherapy, the presence of nonpulmonary visceral metastasis, and primary mediastinal NSGCT as significant and independent prognostic factors for progression and survival. In 660 patients with advanced seminoma, only the presence of nonpulmonary visceral metastasis was an important predictor of progression and survival (International Germ Cell Consensus Classification, 1997).
Based on these analyses the International Germ Cell Cancer Collaborative Group (1997) (IGCCCG) risk classification for advanced GCT was developed (Table 31–4). The IGCCCG risk group should be determined for each patient with metastatic GCT and this should be used to guide treatment decision making on the choice of chemotherapy (discussed later). This classification applies only to patients with advanced GCT at the time of diagnosis and is not applicable to patients with relapsed GCT. It is also based on the postorchiectomy serum tumor marker levels at the start of chemotherapy not the preorchiectomy levels.
Table 31–4 International Germ Cell Cancer Collaborative Group Risk Classification for Advanced GCT
| Good Prognosis | |
|---|---|
| NONSEMINOMA | SEMINOMA |
| Testicular/retroperitoneal primary | Any primary site |
| and | and |
| No nonpulmonary visceral metastases | No nonpulmonary visceral metastases |
| and | and |
| Good markers—all of: | Normal AFP, any hCG, any LDH |
| AFP <1000 ng/mL and | |
| hCG <5000 IU/L (1000 ng/mL) and | |
| LDH <1.5 × upper limit of normal | |
| 56% of nonseminomas | 90% of seminomas |
| 5-year PFS 89% | 5-year PFS 82% |
| 5-year survival 92% | 5-year survival 86% |
| Intermediate Prognosis | |
| NONSEMINOMA | SEMINOMA |
| Testicular/retroperitoneal primary | Any primary site |
| and | and |
| No nonpulmonary visceral metastases | Nonpulmonary visceral metastases |
| and | and |
| Intermediate markers—any of: | Normal AFP, any hCG, any LDH |
| AFP ≥1000-10,000 ng/mL and ≤10,000 ng/mL or | |
| hCG ≥5000-50,000 IU/L and ≤50,000 IU/L or | |
| LDH ≥1.5 × N and ≤10 × N | |