Neoplasms of the Prostate Gland: Introduction
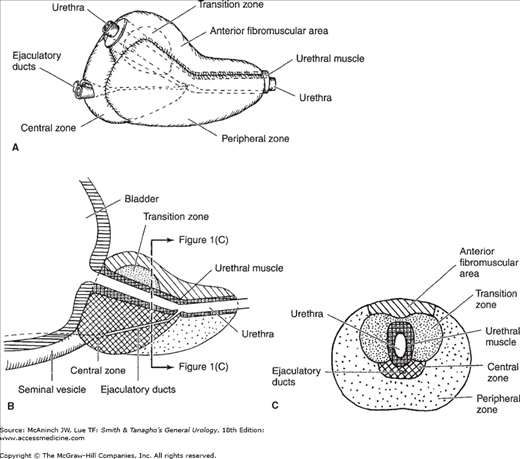
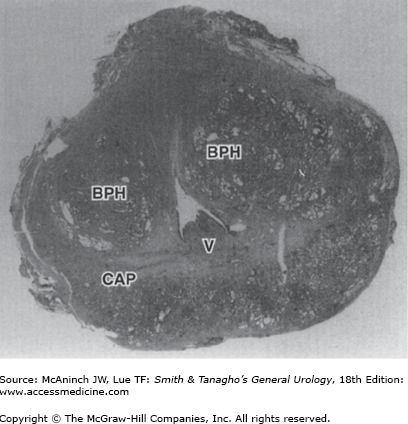
The prostate gland is the male organ most commonly afflicted with either benign or malignant neoplasms. McNeal et al (1988) popularized the concept of zonal anatomy of the prostate. Three distinct zones have been identified (Figure 23–1). The peripheral zone accounts for 70% of the volume of the young adult prostate, the central zone accounts for 25%, and the transition zone accounts for 5%. These anatomic zones have distinct ductal systems but, more important, are differentially afflicted with neoplastic processes. Sixty to seventy percent of carcinomas of the prostate (CaP) originate in the peripheral zone, 10–20% in the transition zone, and 5–10% in the central zone (McNeal et al, 1988). Benign prostatic hyperplasia (BPH) uniformly originates in the transition zone (Figure 23–2).
Benign Prostatic Hyperplasia
BPH is the most common benign tumor in men, and its incidence is age related. The prevalence of histologic BPH in autopsy studies rises from approximately 20% in men aged 41–50, to 50% in men aged 51–60, and to >90% in men older than 80 years. Although clinical evidence of disease occurs less commonly, symptoms of prostatic obstruction are also age related. At age 55, approximately 25% of men report obstructive voiding symptoms. At age 75, 50% of men complain of a decrease in the force and caliber of their urinary stream.
Risk factors for the development of BPH are poorly understood. Some studies have suggested a genetic predisposition, and some have noted racial differences. Approximately 50% of men younger than 60 years who undergo surgery for BPH may have a heritable form of the disease. This form is most likely an autosomal dominant trait, and first-degree male relatives of such patients carry an increased relative risk of approximately fourfold.
The etiology of BPH is not completely understood, but it seems to be multifactorial and endocrine controlled. The prostate is composed of both stromal and epithelial elements, and each, either alone or in combination, can give rise to hyperplastic nodules and the symptoms associated with BPH. Each element may be targeted in medical management schemes.
Observations and clinical studies in men have clearly demonstrated that BPH is under endocrine control. Castration results in the regression of established BPH and improvement in urinary symptoms. Additional investigations have demonstrated a positive correlation between levels of free testosterone and estrogen and the volume of BPH. The latter may suggest that the association between aging and BPH might result from the increased estrogen levels of aging causing induction of the androgen receptor, which thereby sensitizes the prostate to free testosterone. There is evidence that estrogens acting through stromal and epithelial estrogen receptors may contribute, in part, to diseases of the prostate. Genetic or environmental factors that influence 5α-reductase appear to be important in the development of BPH as well (Alan et al, 2008; Gail et al, 2008).
As discussed earlier, BPH develops in the transition zone. It is truly a hyperplastic process resulting from an increase in cell number. Microscopic evaluation reveals a nodular growth pattern that is composed of varying amounts of stroma and epithelium. Stroma is composed of varying amounts of collagen and smooth muscle. The differential representation of the histologic components of BPH explains, in part, the potential responsiveness to medical therapy. Thus, α-blocker therapy may result in excellent responses in patients with BPH that has a significant component of smooth muscle, while those with BPH predominantly composed of epithelium might respond better to 5α-reductase inhibitors. Patients with significant components of collagen in the stroma may not respond to either form of medical therapy. Unfortunately, one cannot reliably predict responsiveness to a specific therapy (see later).
As BPH nodules in the transition zone enlarge, they compress the outer zones of the prostate, resulting in the formation of a so-called surgical capsule. This boundary separates the transition zone from the peripheral zone and serves as a cleavage plane for open enucleation of the prostate during open simple prostatectomies performed for BPH.
One can relate the symptoms of BPH to either the obstructive component of the prostate or the secondary response of the bladder to the outlet resistance. The obstructive component can be subdivided into the mechanical and the dynamic obstruction.
As prostatic enlargement occurs, mechanical obstruction may result from intrusion into the urethral lumen or bladder neck, leading to a higher bladder outlet resistance. Prior to the zonal classification of the prostate, urologists often referred to the “three lobes” of the prostate, namely, the median and the two lateral lobes. Prostatic size on digital rectal examination (DRE) correlates poorly with symptoms, in part, because the median lobe is not readily palpable.
The dynamic component of prostatic obstruction explains the variable nature of the symptoms experienced by patients. The prostatic stroma, composed of smooth muscle and collagen, is rich in adrenergic nerve supply. The level of autonomic stimulation thus sets a tone to the prostatic urethra. Use of α-blocker therapy decreases this tone, resulting in a decrease in outlet resistance.
The irritative voiding complaints (see later) of BPH result from the secondary response of the bladder to the increased outlet resistance. Bladder outlet obstruction leads to detrusor muscle hypertrophy and hyperplasia as well as collagen deposition. Although the latter is most likely responsible for a decrease in bladder compliance, detrusor instability is also a factor. On gross inspection, thickened detrusor muscle bundles are seen as trabeculation on cystoscopic examination. If left unchecked, mucosal herniation between detrusor muscle bundles ensues, causing diverticula formation (so-called false diverticula composed of only mucosa and serosa).
The symptoms of BPH can be divided into obstructive and irritative complaints. Obstructive symptoms include hesitancy, decreased force and caliber of stream, sensation of incomplete bladder emptying, double voiding (urinating a second time within 2 hours of the previous void), straining to urinate, and postvoid dribbling. Irritative symptoms include urgency, frequency, and nocturia.
The self-administered questionnaire originally developed by the American Urological Association (AUA) is both valid and reliable in identifying the need to treat patients and in monitoring their response to therapy. The AUA Symptom Score Questionnaire has been extensively validated and translated, and it is now more commonly called the International Prostate Symptom Score (IPSS) (Table 23–1). The IPSS is perhaps the single most important tool used in the evaluation of patients with BPH and is recommended for all patients before the initiation of therapy. This assessment focuses on seven items that ask patients to quantify the severity of their obstructive or irritative complaints on a scale of 0–5. Thus, the score can range from 0 to 35. An IPSS of 0–7 is considered mild, 8–19 is considered moderate, and 20–35 is considered severe. The relative distribution of scores for BPH patients and control subjects is, respectively, 20% and 83% in those with mild scores, 57% and 15% in those with moderate scores, and 23% and 2% in those with severe scores (McConnell et al, 1994). As with other quality-of-life surveys, a reasonable degree of both literacy and numeracy is necessary for valid results. A multimedia version of the IPSS has been proposed which is more reliable among low-education populations (Bryant et al, 2009).
AUA Score | |||||||
|---|---|---|---|---|---|---|---|
Urinary symptoms (symptom score criteria) | Not at all | Less than 1 time in 5 | Less than half the time | About half the time | More than half the time | Almost always | |
1. Incomplete emptying Over the past month, how often have you had a sensation of not emptying your bladder completely after you finished urinating? | 0 | 1 | 2 | 3 | 4 | 5 | |
2. Frequency Over the past month, how often have you had to urinate again less than 2 hours after you finished urinating? | 0 | 1 | 2 | 3 | 4 | 5 | |
3. Intermittency Over the past month, how often have you found you stopped and started again several times when you urinate? | 0 | 1 | 2 | 3 | 4 | 5 | |
4. Urgency Over the past month, how often have you found it difficult to postpone urination? | 0 | 1 | 2 | 3 | 4 | 5 | |
5. Weak stream Over the past month, how often have you had a weak urinary stream? | 0 | 1 | 2 | 3 | 4 | 5 | |
6. Straining Over the past month, how often have you had to push or strain to begin urination? | 0 | 1 | 2 | 3 | 4 | 5 | |
None | 1 time | 2 times | 3 times | 4 times | 5 or more times | ||
7. Nocturia Over the past month, how many times did you most typically get up to urinate from the time you went to bed at night until the time you got up in the morning? | 0 | 1 | 2 | 3 | 4 | 5 | |
International Prostate Symptom Score (IPSS) = sum of questions A1 to A7 | |||||||
Quality of life due to urinary problems | |||||||
Delighted | Pleased | Mostly satisfied | Mixed—about equally satisfied and unsatisfied | Mostly dissatisfied | Unhappy | Terrible | |
If you were to spend the rest of your life with your urinary condition just the way it is now, how would you feel about that? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
A detailed history focusing on the urinary tract excludes other possible causes of symptoms that may not result from the prostate, such as urinary tract infection, neurogenic bladder, urethral stricture, or prostate cancer.
A physical examination, DRE, and focused neurologic examination are performed on all patients. The size and consistency of the prostate is noted, even though prostate size, as determined by DRE, does not correlate with severity of symptoms or degree of obstruction. BPH usually results in a smooth, firm, elastic enlargement of the prostate. Induration, if detected, must alert the physician to the possibility of cancer and the need for further evaluation (ie, prostate-specific antigen [PSA], transrectal ultrasound [TRUS], and biopsy).
A urinalysis to exclude infection or hematuria and serum creatinine measurement to assess renal function are required. Renal insufficiency may be observed in 10% of patients with prostatism and warrants upper-tract imaging. Patients with renal insufficiency are at an increased risk of developing postoperative complications following surgical intervention for BPH. Serum PSA is considered optional, but most physicians will include it in the initial evaluation. PSA, compared with DRE alone, certainly increases the ability to detect CaP, but because there is much overlap between levels seen in BPH and CaP, its use remains controversial (see Section “Prostate Cancer Screening and Chemoprevention”).
Upper-tract imaging (renal ultrasound or computed tomography [CT] urogram) is recommended only in the presence of concomitant urinary tract disease or complications from BPH (eg, hematuria, urinary tract infection, renal insufficiency, history of stone disease). TRUS is useful to determine prostate size for men planning to undergo prostate surgery who are suspected to have severe prostate enlargement based on DRE.
Cystoscopy is not routinely recommended to determine the need for treatment but may assist in choosing the surgical approach in patients opting for invasive therapy. When marked obstructive symptoms exist in the setting of relative minimal prostate enlargement, cystoscopy may be useful to identify a high bladder neck, urethral stricture, or other pathology. If BPH is associated with hematuria, then cystoscopy is mandatory to rule out other bladder pathology.
Measurement of flow rate, determination of postvoid residual urine, and pressure-flow studies are considered optional. Cystometrograms and urodynamic profiles are reserved for patients with suspected neurologic disease or those who have failed prostate surgery.
Other obstructive conditions of the lower urinary tract, such as urethral stricture, bladder neck contracture, bladder stone, or CaP, must be entertained when evaluating men with presumptive BPH. A history of previous urethral instrumentation, urethritis, or trauma should be elucidated to exclude urethral stricture or bladder neck contracture. Hematuria and pain are commonly associated with bladder stones. CaP may be detected by abnormalities on the DRE or an elevated PSA (see later).
A urinary tract infection, which can mimic the irritative symptoms of BPH, can be readily identified by urinalysis and culture; however, a urinary tract infection can also be a complication of BPH. Although irritative voiding complaints are also associated with carcinoma of the bladder, especially carcinoma in situ, the urinalysis usually shows evidence of hematuria. Likewise, patients with neurogenic bladder disorders may have many of the signs and symptoms of BPH, but a history of neurologic disease, stroke, diabetes mellitus, or back injury may be present as well. In addition, examination may show diminished perineal or lower extremity sensation or alterations in rectal sphincter tone or the bulbocavernosus reflex. Simultaneous alterations in bowel function (constipation) might also alert one to the possibility of a neurologic origin.
After patients have been evaluated, they should be informed of the various therapeutic options for BPH. It is advisable for patients to consult with their physicians to make an educated decision on the basis of the relative efficacy and side effects of the treatment options.
Specific treatment recommendations can be offered for certain groups of patients. For those with mild symptoms (IPSS score, 0–7), watchful waiting is generally advised. On the other end of the therapeutic spectrum, absolute surgical indications include urinary retention refractory to medical management and attempts at catheter removal, recurrent urinary tract infection, recurrent gross hematuria, bladder stones, renal insufficiency, or large bladder diverticula.
Very few studies on the natural history of BPH have been reported. The risk of progression or complications is uncertain. However, in men with symptomatic BPH, it is clear that progression is not inevitable and that some men undergo spontaneous improvement or resolution of their symptoms.
Retrospective studies on the natural history of BPH are inherently subject to bias, related to patient selection and the type and extent of follow-up. Very few prospective studies addressing the natural history of BPH have been reported. A large randomized study compared finasteride with placebo in men with moderately to severely symptomatic BPH and enlarged prostates on DRE (McConnell et al, 1998). Patients in the placebo arm of the study had a 7% risk of developing urinary retention over 4 years.
As mentioned earlier, watchful waiting is the appropriate management of men with mild symptom scores (0–7). Men with moderate or severe symptoms can also be managed in this fashion if they so choose. Neither the optimal interval for follow-up nor specific end points for intervention have been defined.
The human prostate and bladder base contains α1-adrenoreceptors, and the prostate shows a contractile response to corresponding agonists. The contractile properties of the prostate and bladder neck seem to be mediated primarily by the subtype α1a-receptors. α-Blockade has been shown to result in both objective and subjective degrees of improvement in the symptoms and signs of BPH in some patients. α-Blockers can be classified according to their receptor selectivity as well as their half-life (Table 23–2).
Classification | Oral dosage |
|---|---|
α–Blockers | |
Nonselective | |
Phenoxybenzamine | 10 mg twice a day |
α1, short acting | |
Prazosin | 2 mg twice a day |
α1, long acting | |
Terazosin | 5 or 10 mg daily |
Doxazosin | 4 or 8 mg daily |
α1a-Selective | |
Tamsulosin | 0.4 or 0.8 mg daily |
Alfuzosin | 10 mg daily |
Silodosin | 8 mg daily |
5α-Reductase inhibitors | |
Finasteride | 5 mg daily |
Dutasteride | 0.5 mg daily |
Phenoxybenzamine and prazosin are the prototypical nonselective and selective α-blockers, but today, they are primarily of historical interest.
Long-acting α1-blockers make once-a-day dosing possible, but dose titration is still necessary. Terazosin is initiated at 1 mg daily for 3 days and increased to 2 mg daily for 11 days and then to 5 mg/d. Dosage can be escalated to 10 mg daily if necessary. Therapy with doxazosin is started at 1 mg daily for 7 days and increased to 2 mg daily for 7 days, and then to 4 mg daily. Dosage can be escalated to 8 mg daily if necessary. Possible side effects include orthostatic hypotension, dizziness, tiredness, retrograde ejaculation, rhinitis, and headache.
Selective blockade of the α1a-receptors, which are localized in the prostate and bladder neck, results in fewer systemic (particularly cardiovascular) side effects, thus obviating the need for dose titration with these agents (tamsulosin, alfuzosin, and silodosin). Other side effects such as retrograde ejaculation still can occur.
Several randomized, double-blind, placebo-controlled trials, individually comparing α-blockers with placebo, have demonstrated the safety and efficacy of all of these agents.
Finasteride is a 5α-reductase inhibitor that blocks the conversion of testosterone to dihydrotestosterone (DHT). This drug affects the epithelial component of the prostate, resulting in a reduction in the size of the gland and improvement in symptoms. Six-month therapy is required to see the maximum effects on prostate size (20% reduction) and symptomatic improvement.
Several randomized, double-blind, placebo-controlled trials have compared finasteride with placebo. Efficacy, safety, and durability are well established. However, symptomatic improvement is seen only in men with enlarged prostates (>40 cm3). Side effects are uncommon and include decreased libido, decreased ejaculate volume, and impotence. Serum PSA is reduced by approximately 50% in patients being treated with finasteride, but individual values may vary.
Dutasteride differs from finasteride as it inhibits both isoenzymes of 5α-reductase. Similar to finasteride, it reduces serum PSA and total prostate volume. Randomized, placebo-controlled trials have shown the efficacy of dutasteride in symptomatic relief, symptoms scores, peak urinary flow rate, and reduced risk of acute urinary retention and the need for surgery. Side effects are relatively uncommon and include erectile dysfunction, decreased libido, gynecomastia, and ejaculation disorders. Few studies comparing finasteride and dutasteride head-to-head. One retrospective analysis of >5000 men older than 65 years treated with 5α-reductase inhibitors in the mid-2000s found small but statistically significantly differences, with rates of urinary retention of 12% and 14.7% for dutasteride and finasteride, respectively (p= 0.0042), and rates of prostate surgery of 3.9% and 5.1%, respectively (p= 0.03) (Fenter et al, 2008).
The first randomized, double-blind, placebo-controlled study investigating combination α-blocker and 5α-reductase inhibitor therapy was a four-arm Veterans Administration Cooperative Trial comparing placebo, finasteride alone, terazosin alone, and combination finasteride and terazosin (Lepor et al, 1996). More than 1200 patients participated, and significant decreases in IPSS and increases in urinary flow rates were seen only in the arms containing terazosin. However, one must note that enlarged prostates were not an entry criterion; in fact, prostate size in this study was much smaller than that in previous controlled trials using finasteride (32 vs 52 cm3). McConnell and colleagues conducted a long-term, double-blind trial involving 3047 men to compare the effects of placebo, doxazosin, finasteride, and combination therapy on measures of the clinical progression of BPH (McConnell et al, 2003). The risk of overall clinical progression—defined as an increase above baseline of at least four points in the IPSS, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection—was significantly reduced by doxazosin (39% risk reduction) and finasteride (34% risk reduction), as compared with placebo. The reduction in risk associated with combination therapy (66% risk reduction) was significantly greater than that associated with doxazosin or finasteride alone. Patients most likely to benefit from combination therapy are those in whom baseline risk of progression is very high, generally patients with larger glands and higher PSA values.
Phytotherapy refers to the use of plants or plant extracts for medicinal purposes. The use of phytotherapy in BPH has been popular in Europe for years, and its use in the United States is growing as a result of patient-driven enthusiasm. Several plant extracts have been popularized, including the saw palmetto berry (Serenoa repens), the bark of Pygeum africanum, the roots of Echinacea purpurea and Hypoxis rooperi, pollen extract, and the leaves of the trembling poplar. S. repens has been the most well-studied agent, usually at 320 mg/day. Given the poor regulation of the nutritional supplement industry, actual tablet content may vary extremely from the dose noted on the product label (Feifer et al, 2002). A prospective, randomized clinical trial of saw palmetto showed no benefit beyond placebo for either IPSS improvement or urinary flow rate (Bent et al, 2006). An updated systematic review including this and other trials confirmed no improvement over placebo for this approach (Wilt et al, 2009).
The vast majority of subtotal prostatectomies undertaken for BPH can be completed endoscopically. Most of these procedures involve the use of a spinal or general anesthetic and usually require an overnight hospital stay. Magnitude and durability of IPSS and flow rate improvement with transurethral resection of the prostate (TURP) is superior to that of any minimally invasive therapy. However, the length of hospital stay of patients undergoing TURP is greater. Risks of TURP include retrograde ejaculation (75%), impotence (5–10%), and incontinence (<1%). Complications include bleeding; urethral stricture or bladder neck contracture; perforation of the prostate capsule with extravasation; and, if severe, transurethral resection (TUR) syndrome resulting from a hypervolemic, hyponatremic state due to absorption of the hypotonic irrigating solution.
Clinical manifestations of the TUR syndrome include nausea, vomiting, confusion, hypertension, bradycardia, and visual disturbances. The risk of the TUR syndrome increases with resection times >90 minutes and is usually seen in older men. Treatment includes diuresis and, in severe cases, hypertonic saline administration. TURP can now be performed with a bipolar electrode, allowing resection to be performed under saline irrigation. This approach eliminates the hyponatremia responsible for TUR syndrome, though significant fluid volume absorption can still occur with prolonged resection.
Men with moderate to severe symptoms and a small prostate often have posterior commissure hyperplasia (elevated bladder neck). These patients will often benefit from an incision of the prostate. This procedure is more rapid and less morbid than TURP. Outcomes in well-selected patients are comparable, although a lower rate of retrograde ejaculation with transurethral incision has been reported (25%). The technique involves two incisions using the Collins knife at the 5- and 7-o’clock positions. The incisions are started just distal to the ureteral orifices and are extended outward to the verumontanum.
Increasingly popular in recent years, ablative techniques use photo- or electroevaporation to ablate obstructing prostate tissue. The two most commonly used devices for these procedures are the neodymium-doped yttrium-aluminum-garnet (Nd:YAG) KTP “GreenLight” laser, which is preferentially absorbed by hemoglobin, and the plasma vaporization “Button” electrode. The latter works with a standard contemporary bipolar generator used for bipolar TURP.
As with modern TURP, these procedures are performed under saline irrigation. The goal of the procedure in either case is to produce a central prostate defect comparable with what would be expected after a traditional TURP, but with less bleeding and lower risk of perforation. The potential downsides are greater irritative voiding symptoms in the short term after the procedure and less durability of the result than a standard TURP. Also, as tissue is destroyed rather than resected, no specimen is sent to pathology for review.
Rather than progressive resection or ablation of tissue from the urethra outward as with TURP and its derivatives, HoLEP denotes an anatomic dissection in the plane between the central and peripheral zones of the prostate. This approach is felt to provide the largest defect and perhaps the longest durability, but entails a longer learning curve than TURP or TUVP.
When the prostate is too large to be removed endoscopically, an open enucleation is necessary. What constitutes “too large” is subjective and will vary depending upon the surgeon’s experience with TURP. Glands >100 g are usually considered for open enucleation. Open prostatectomy may also be initiated when concomitant bladder diverticulum or a large bladder stone is present or if dorsal lithotomy positioning is not possible.
Open prostatectomies can be done with either a suprapubic or retropubic approach. A simple suprapubic prostatectomy is performed transvesically and is the operation of choice in dealing with concomitant bladder pathology. After the bladder is opened, a semicircular incision is made in the bladder mucosa, distal to the trigone. The dissection plane is initiated sharply, and then blunt dissection with the finger is performed to remove the adenoma. The apical dissection should be done sharply to avoid injury to the distal sphincteric mechanism. After the adenoma is removed, hemostasis is attained with suture ligatures, and both a urethral and a suprapubic catheter are inserted before closure. In a simple retropubic prostatectomy, the bladder is not entered. Rather, a transverse incision is made in the surgical capsule of the prostate, and the adenoma is enucleated as described earlier. Only a urethral catheter is needed at the end of the procedure. Robot-assisted simple prostatectomy has been reported in recent small series (Sutherland et al, 2011).
Microwave hyperthermia is most commonly delivered with a transurethral catheter. Some devices cool the urethral mucosa to decrease the risk of injury. However, if temperatures are not >45°C, cooling is unnecessary. Improvement in IPSS and flow rate has been documented, but as these procedures are done in the office with no visual verification of tissue ablation, results have been mixed. Strong financial incentives, however, have driven frequent utilization in certain clinical contexts.
Very sparse prospective data are available to fairly compare any of the above procedures with TURP or with each other. A recent meta-analysis found few differences, but the component studies tended to be small and with limited follow-up (Ahyai et al, 2010). All of the newer procedures are more expensive than TURP, and comparative cost-effectiveness studies are sorely needed.
Carcinoma of the Prostate
Prostate cancer is the most common noncutaneous cancer detected among American men. More than 200,000 cases are detected annually (Jemal et al, 2010). Approximately 30,000 men die of the disease annually—more than any other tumor type except lung cancer. However, prostate cancer mortality at the population level has declined by roughly 40% since the mid-1990s, during a time in which men have been living longer and therefore have been more likely to reach the older ages at which prostate cancer mortality would be expected to increase. There are no known dietary or other environmental trends that can explain this decline in mortality rates. The explanation is controversial but is likely multifactorial, reflecting a combination of screening programs and improvements in treatment.
These improvements in prostate cancer mortality have come at the cost of significant rates of overdiagnosis and overtreatment. The number of prostate cancer deaths annually is far outweighed by the number of diagnoses, and most men diagnosed ultimately die of other causes, most often cardiovascular disease. Of all cancers, the prevalence of CaP increases the most rapidly with age. However, unlike most cancers, which have a peak age of incidence, the incidence of CaP continues to increase with advancing age. The lifetime risk of a 50-year-old man for latent CaP (ie, detected as an incidental finding at autopsy, not related to the cause of death) is 40%; for lifetime diagnosis of CaP, 15%; and for death from CaP, 2.9%. Thus, many prostate cancers are indolent and inconsequential to the patient while others are virulent, and if detected too late or left untreated, they result in a patient’s death. This broad spectrum of biological activity can make decision making for individual patients difficult, and highlight the critical need for risk stratification of prostate cancers, which will be discussed in further detail later.
Several risk factors for prostate cancer have been identified. As discussed earlier, increasing age heightens the risk for CaP. Which of the factors associated with the aging process are responsible for this observation is unknown. The probability of CaP diagnosis in a man younger than 40 years is 1 in 10,000; for men 40–59 years old, it is 1 in 103; and for men 60–79 years old, it is 1 in 8. African Americans are at a higher risk for CaP than whites. In addition, African American men tend to present with higher disease risk than whites. Controversial data have been reported suggesting that mortality from this disease may also be higher for African Americans. A positive family history of CaP also increases the relative risk for CaP. The age of disease onset in the family member with the diagnosis of CaP affects a patient’s relative risk. If the age of onset is 70, the relative risk is increased fourfold; if the age of onset is 60, the relative risk is increased fivefold; and if the age of onset is 50, the relative risk is increased sevenfold.
Although diagnostic biases due to varying penetrance of PSA screening exist across countries, differences in the incidence of prostate cancer are real. These differences may be related, in part, to differences in diet (Chan et al, 2005). Epidemiologic studies have shown that the incidence of clinically significant prostate cancer is much lower in parts of the world where people eat a predominantly low fat, plant-based diet. In addition, migrant studies demonstrate that when men from a low-risk country move to the United States and begin eating a westernized diet, their rates of prostate cancer increase severalfold and approach that of the host country. Total fat intake, animal fat intake, and red meat intake are associated with an increased risk of prostate cancer, whereas intake of fish is associated with a decreased risk. There is considerable controversy on the impact of obesity on prostate cancer. Some studies suggest that obesity is associated with an increased risk of more advanced disease and a higher recurrence rate after treatment. In addition, lycopene, selenium, omega-3 fatty acids (fish), and vitamin E intake have been shown to be protective, whereas vitamin D and calcium increase risk. No dietary supplementation study has yet shown a tangible benefit in terms of reducing risk of diagnosis or mortality. Previous vasectomy has been suggested as a factor that heightens the risk for CaP, but this association has not been validated in larger studies (Cox et al, 2002).
More than 95% of the prostate cancers are adenocarcinomas. The histology of the remaining 5% of prostate cancer is heterogeneous, arising from stromal, epithelial, or ectopic cells. Nonadenocarcinoma variants can be categorized into two groups based on the cellular origin: epithelial and nonepithelial. Epithelial variants consist of endometrioid, mucinous, signet-ring, adenoid cystic, adenosquamous, squamous cell, transitional cell, neuroendocrine, and comedocarcinoma. Nonepithelial variants include rhabdomyosarcoma, leiomyosarcoma, osteosarcoma, angiosarcoma, carcinosarcoma, malignant lymphoma, and metastatic neoplasms among others.
The remainder of this discussion will focus on adenocarcinoma. However, it is increasingly evident that neuroendocrine (“small cell”) differentiation may occur in response to prolonged androgen deprivation. This can be recognized by staining such tissue for neuroendocrine markers (chromogranin A, neuron-specific enolase) and/or by measuring such markers in serum.
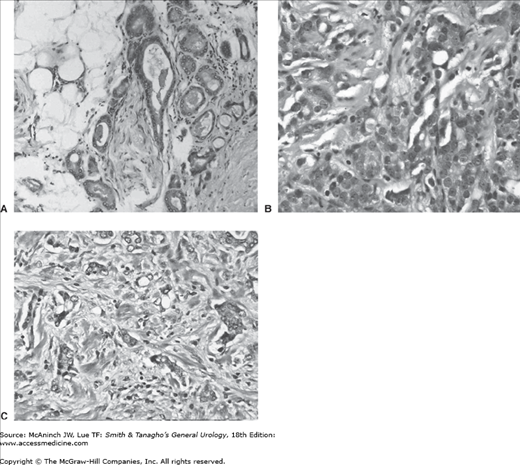
The cytologic characteristics of CaP include hyperchromatic, enlarged nuclei with prominent nucleoli (Figure 23–3). Cytoplasm is often abundant; thus, nuclear-to-cytoplasmic ratios are not often helpful in making a diagnosis of CaP, unlike their usefulness in diagnosing many other neoplasms. Cytoplasm is often slightly blue tinged or basophilic, which may assist in the diagnosis. The diagnosis of CaP is truly an architectural one. The basal cell layer is absent in CaP, whereas it is present in normal glands, BPH glands, and the precursor lesions of CaP. If the diagnosis of CaP is in question, high-molecular-weight keratin immunohistochemical staining is useful, as it preferentially stains basal cells. Absence of staining is thus consistent with CaP. Those biopsies that remain equivocal could be stained with new markers such as AMACR or EPCA, which appear to identify those with the disease, but who have equivocal or negative biopsies based on standard tissue staining.
Figure 23–3.
Gleason primary grade 3 (A), grade 4 (B), and grade 5 (C) cancer (200×). A: Glands are well developed with variation in contour and morphology. The glands grow in an infiltrative pattern. Nuclear features of malignancy include mild nuclear enlargement, granular chromatin, and nucleoli. B: Malignant cells have trabecular, glandular, and infiltrative growth pattern forming small solid nests and abortive, fused glandular lumens. Malignant nuclear features include marked nuclear enlargement and macronucleoli. C: Highly infiltrative growth pattern with single cells and small nests of malignant epithelial cells. Cytologic features include marked nuclear pleomorphism and anisonucleosis with irregular contours, coarse irregular chromatin distribution, and macronucleoli.
Prostatic intraepithelial neoplasia (PIN) and atypical small acinar proliferation (ASAP) are thought to be precursor lesions. However, the risk of prostate cancer appears to be higher in those with the latter histology. Men found to have either lesion may be at an increased risk of prostate cancer and warrant repeat biopsy, particularly if an extended-core biopsy was not performed initially. High-grade PIN (HGPIN) is characterized by cellular proliferations within preexisting ducts and glands, with nuclear and nucleolar enlargement similar to prostate cancer. However, unlike cancer, HGPIN retains a basal cell layer identifiable by immunohistochemistry.
Approximately 60–70% of cases of CaP originate in the peripheral zone, 10–20% originate in the transition zone, and 5–10% in the central zone. Although prostate cancer is frequently multifocal, the use of widespread screening and extended biopsy techniques has resulted in the increasing detection of unifocal and smaller cancers.
Penetration of the prostatic capsule by cancer is a common event and often occurs along perineural spaces. Seminal vesicle invasion is associated with a high likelihood of regional or distant disease. Locally advanced CaP may invade the bladder trigone, resulting in ureteral obstruction. Rectal involvement is rare as Denonvilliers’ fascia represents a strong barrier. (Of note, this barrier is largely one way, as rectal cancer may invade the prostate relatively commonly.) Lymphatic metastases are most often identified in the obturator, external iliac, and internal lymph node chains. Other sites of nodal involvement include the common iliac, presacral, and periaortic lymph nodes.
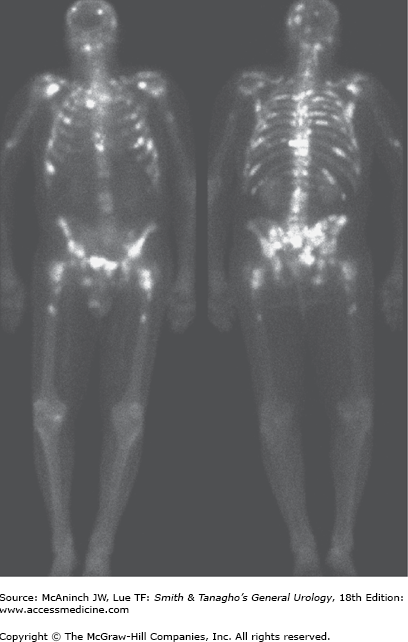
The axial skeleton is the most usual site of distant metastases, with the lumbar spine being most frequently implicated (Figure 23–4). The next most common sites in decreasing order are proximal femur, pelvis, thoracic spine, ribs, sternum, skull, and humerus. The bone lesions of metastatic CaP are typically osteoblastic. Involvement of long bones can lead to pathologic fractures. Vertebral body involvement with significant tumor masses extending into the epidural space can result in cord compression. Visceral metastases most commonly involve the lung, liver, and adrenal gland. Central nervous system involvement is usually a result of direct extension from skull metastasis.
Molecular profiling of human tissues has identified differential expression of specific genes and proteins in the progression from normal precursor tissue to preneoplastic lesions to cancer (both androgen dependent and independent). In doing so, diagnostic, prognostic, and therapeutic markers have been discovered.
Chromosomal rearrangements or copy number abnormalities at 8p, 10q, 11q, 13q, 16q, 17p, and 18q have been described in prostate cancers. Some of these such as specific loss at 8p23.2 and/or gain at 11q13.1 are predictive of prostate cancer progression.
The entire prostate microenvironment, not just the epithelial compartment, is important for both normal and neoplastic growth as significant epithelial–mesenchymal/stromal interactions occur (Chung et al, 2005). Molecular events may not always be spontaneous, but the product of environmental influences. For instance, both epidemiologic and molecular data suggest that inflammation may be related to prostate cancer development (Nelson et al, 2004). RNASEL, encoding an interferon-inducible ribonuclease, and MSR1, encoding subunits of the macrophage scavenger receptor, are candidate-inherited susceptibility genes for prostate cancer, including familial cancer. Proliferative inflammatory atrophy lesions containing activated inflammatory cells and proliferating epithelial cells appear likely to be precursors to PIN lesions and prostatic carcinomas.
Using a novel bioinformatics approach, Tomlins and colleagues identified two transcription factors ERG and EtV1 that were overexpressed in prostate cancer tissue. Furthermore, TMPRSS2 was fused to these genes suggesting that fusion accounted for overexpression. This genetic rearrangement appears to be the most common identified in prostate cancer. The TMPRSS2:ERG fusion has been identified in approximately 50% of prostate tumors and likely represents an early molecular event in carcinogenesis. Furthermore, this fusion may yield a distinct phenotype with a more aggressive natural history, independent of Gleason grade (Narod et al, 2008).
Some overexpressed genes or combinations of genes may be important biomarkers capable of not only identifying cancer in equivocal biopsy samples (alpha-methylacyl coenzyme A racemase [AMACR] and early prostate cancer antigen [EPCA]) but also in predicting response to treatment and progression (Rubin, 2004). Multiple research efforts have identified other promising multiparametric models to improve risk stratification and prediction (Cuzick et al, 2011; Mucci et al, 2008; Paris et al, 2010; Penney et al, 2011), though none of these has yet been well validated or reached clinical practice. Beyond genetic analyses, parallel advances in proteomics and metabolomics likewise are yielding novel insights into both the pathophysiology of prostate cancer and improved risk stratification of the disease (Sreekumar et al, 2009).
The number of prostate cancers attributable to heritable factors may be greater than once thought (Lichtenstein et al, 2000). Although the loci 8q, 3p, 7p/q, 9q, 10q, 11q, 17q, and 22q have been identified as harboring potential predisposition genes in those with a family history of prostate cancer, a multigene model may best explain familial clustering of this disease. In addition, men with a family history of breast and/or ovarian cancer may be offered a predictive genetic test to determine whether or not they carry the family specific BRCA1/2 mutations as they are at increased risk of breast and prostate cancers.
The large majority of patients with early-stage CaP are asymptomatic. The presence of symptoms often suggests locally advanced or metastatic disease. Obstructive or irritative voiding complaints can result from local growth of the tumor into the urethra or bladder neck or from its direct extension into the trigone of the bladder. Much more commonly, however, such symptoms are attributable to coexisting BPH. Metastatic disease to the bones may cause bone pain. Metastatic disease to the vertebral column with impingement on the spinal cord may be associated with symptoms of cord compression, including paresthesias and weakness of the lower extremities and urinary or fecal incontinence.
A physical examination, including a DRE, is needed. Induration or nodularity, if detected, must alert the physician to the possibility of cancer and the need for further evaluation (ie, PSA, TRUS, and biopsy). Locally advanced disease with bulky regional lymphadenopathy may lead to lymphedema of the lower extremities. Specific signs of cord compression relate to the level of the compression and may include weakness or spasticity of the lower extremities and a hyperreflexic bulbocavernosus reflex.
Azotemia can result from bilateral ureteral obstruction either from direct extension into the trigone or from retroperitoneal adenopathy. Anemia may be present in metastatic disease. Alkaline phosphatase may be elevated in the presence of bone metastases. Serum acid phosphatase may be elevated with disease outside the confines of the prostate.
PSA is a serine protease in the human kallikrein (hK) family produced by benign and malignant prostate tissues. It circulates in the serum as uncomplexed (free or unbound) or complexed (bound) forms. PSA is used both as a diagnostic (screening) tool and as a means of risk-stratifying known prostate cancers. In both contexts, its use is complicated by the fact that PSA is prostate specific, not prostate cancer specific. Other prevalent conditions such as BPH and prostatitis—as well as urethral instrumentation and perineal insults such as prolonged bike ride—can elevate the PSA, producing false-positive results.
A “normal” PSA has traditionally been defined as ≤4 ng/mL, and the positive predictive value of a serum PSA between 4 and 10 ng/mL is approximately 20–30%. For levels in excess of 10 ng/mL, the positive predictive value increases from 42% to 71.4%. In light of variation with age and ethnicity, age- and race-specific reference ranges have been proposed (Oesterling et al, 1993). More importantly, the results of the Prostate Cancer Prevention Trial (PCPT) study, which included biopsy regardless of PSA level—thus avoiding the ascertainment bias otherwise confounding virtually all other studies of PSA—demonstrated that there is no level of PSA below which prostate cancer risk falls to zero. PSA is rather indicative of a continuum of risk—the higher the level, the higher the risk (Thompson et al, 2004).
Current prostate cancer screening and detection strategies therefore include with PSA other risk factors such as family history, race, age, and others (Greene et al, 2009). Online risk calculators integrating these variables have been made generated to determine risk of prostate cancer and risk of high-grade prostate cancer. A calculator based on the PCPT data, for example, is available at http://tinyurl.com/caprisk.
Use of medications such as 5α-reductase inhibitors (including the 1 mg finasteride formulation marked for alopecia as Propecia) must be ascertained, as these medications can artificially lower the PSA by approximately 50%. Interestingly, serum PSA levels have also been noted to be decreased in men with high body mass indexes compared with normal weight men, likely as a result of hemodilution (Banez et al, 2007).
Numerous strategies to refine PSA for cancer detection have been explored. Their common goal in general has been to decrease the number of false-positive test results, thus increasing the specificity and positive predictive value of the test and lead to fewer unnecessary biopsies, lower costs, and reduced morbidity of cancer detection. Attempts at refining PSA have included PSA velocity (PSAV) (change of PSA over time), PSA kinetics (standardizing levels in relation to the size of the prostate), and PSA isoforms (free vs protein-bound molecular forms of PSA).
PSAV refers to the rate of change of serum PSA; its inverse, PSA doubling time (PSADT) indicates the amount of time required for the PSA to double. A retrospective study has shown that men with prostate cancer have a more rapidly rising serum PSA in the years before diagnosis than do men without prostate cancer. Patients whose serum PSA increases by 0.75 ng/mL per year appear to be at an increased risk of harboring cancer. However, PSAV must be interpreted with caution. An elevated PSAV should be considered significant only when several serum PSA assays are carried out by the same laboratory over a period of at least 18 months. Very rapid PSA increases may be indicative of prostatitis (symptomatic or otherwise) rather than cancer. Recent studies have questioned whether PSA kinetics in fact add significantly to the absolute PSA level in the prediagnosis setting (Vickers et al, 2011), and the optimal use of PSA kinetics remains controversial.
PSA levels are elevated on average approximately 0.12 ng/mL per gram of BPH tissue. Thus, patients with enlarged glands due to BPH may have elevated PSA levels. The ratio of PSA to gland volume is termed the PSA density. Some investigators advocate prostate biopsy only if the PSA density exceeds 0.1 or 0.15, while others have not found PSA density to be useful. Problems with this approach include the facts that (1) epithelial–stromal ratios vary from gland to gland and only the epithelium produces PSA and (2) errors in calculating prostatic volume based on TRUS may approach 25%. The positive predictive value of PSA density is slightly higher than the use of a PSA level >4 ng/mL in several series (30–40% vs 20–30%). The other major problem with PSA doubling (PSAD) is that it still requires TRUS, which, while a lower risk procedure than biopsy, is still invasive and uncomfortable. Thus, PSAD may be most useful in settings in which the prostate volume is already known (ie, PSA rising after a negative prior biopsy).
Instead of adjusting the PSA to total prostate volume, some have advocated adjusting it to transition zone volume (PSA transition zone density, PSAT [Djavan et al, 2002]). However, like PSA density, such calculations are subject to error, require TRUS, and do not seem to be superior to the use of PSA in most patients.
Various molecular isoforms of PSA have been identified and studied. Approximately 90% of the serum PSA is bound to α1-antichymotrypsin (ACT), and lesser amounts are free or are bound to α2-macroglobulins. In the latter form, no epitopes to the antibodies used in the current assays are available, while PSA bound ACT may have three of its five epitopes masked. Early studies suggest that prostate cancer patients demonstrate a lower percentage of free PSA than do patients with benign disease. A large multicenter study has reported that in men with a normal DRE and a total PSA level between 4 and 10 ng/mL, a 25% free PSA cutoff would detect 95% of cancers while avoiding 20% of unnecessary biopsies. The cancers associated with >25% free PSA were more prevalent in older patients and generally were less threatening in terms of tumor grade and volume (Catalona et al, 1998). The predictive utility of percentage of free PSA in subsequent studies, however, has been mixed.
More recent studies have focused on other PSA subtypes. A serum panel adding free PSA, intact PSA, and hK2 to total PSA has been shown to improve predictive accuracy for prostate cancer diagnosis among men with a PSA >3 (Vickers et al, JCO 2010) and is currently undergoing validation studies. A truncated form of PSA designated −2proPSA has also shown promise in this setting and is likewise undergoing validation studies (Catalona et al, 2011; Mikolajczyk et al, 2004).
Prostate cancer antigen 3 (PCA3) is a noncoding, prostate-specific mRNA, which is overexpressed in the majority of prostate cancers, with a median 66-fold upregulation compared with adjacent noncancer tissue (Hessels et al, 2007). PCA3 predicts the presence of cancer in a biopsy setting with an accuracy of 74.6% (Groskopf et al, 2006). PCA3 may be particularly useful in the evaluation of men with a negative prior biopsy and a rising PSA (Haese et al, 2008).
Prostate biopsy should be considered in men with an elevated serum PSA, abnormal DRE, or a combination of the two, depending additionally on the patient’s overall health, comorbidities, life expectancy, levels of anxiety and of risk aversion, and information preferences. Prostate biopsy is performed under TRUS guidance using a spring-loaded biopsy device coupled to the imaging probe. Biopsies are taken throughout the peripheral zone of the prostate, with optional additional sampling of any abnormal areas on DRE and/or TRUS. Traditionally, six (sextant) biopsies were taken along a parasagittal line between the lateral edge and the midline of the prostate at the apex, midgland, and base bilaterally. However, several investigators have shown that increasing the number (≥10) and performing more laterally directed biopsies of the peripheral zone will increase detection rates 14–20% over the more traditional sextant technique. Although a small number of prostate cancers will originate in the transition zone, specific transition zone biopsies add little to overall cancer detection rates when an extended-pattern biopsy is performed. Some practitioners do add biopsies of the anterior commissure, a relatively frequent site of initially missed cancers found on second or subsequent biopsy. There is ongoing interest in the use of even more extended biopsy schemes (“saturation biopsy”) or use of a transperineal approach to improve cancer detection, usually in those who have had a negative biopsy, but are thought to be at an increased risk of prostate cancer based on a persistently abnormal serum PSA.
Prostate biopsy is usually performed using local anesthesia and preprocedure antibiotic prophylaxis (usually a fluoroquinolone). The use of local anesthesia, either applied topically along the anterior rectal wall, injected into or adjacent to the prostate, or a combination of the two, decreases pain associated with the procedure. Hematospermia, hematochezia, and hematuria are common occurring in approximately 40–50% of patients. With rising prevalence of antibiotic-resistant bacteria, rates of sepsis despite standard prophylaxis have been growing (Lange et al, 2009). These can be life threatening even in otherwise-healthy men, and patients are counseled to return immediately to the emergency department for any high fevers after the procedure.
Saturation schemes consist of 20 or more cores that emphasize sampling of the peripheral zone. One of the more common saturation schemes involves taking two cores from the lateral base, three cores from the lateral mid, three cores from the apex (including anterior apex), and one core from the parasagittal mid and one core from the parasagittal base. While initial saturation schemes included two cores at both the parasagittal mid and parasagittal base, unique identification of cancer in these areas is rare and thus it has been recommended to only obtain one care for each of these areas. Investigators have demonstrated that saturation biopsies can be performed in the office using a periprostatic block. They observed no improved yield by using a saturation biopsy as the initial biopsy scheme or the first repeat biopsy scheme but rather advocate it as a second repeat biopsy strategy (Jones et al, 2002).
The Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial allows us to prospectively look at both cancer detection rates and quality of cancers in a repeat biopsy situation. Recall that entry criteria for this trial mandated a prior negative biopsy (minimum of a negative sextant biopsy) within 6 months of enrollment. In the placebo arm, 3346 patients underwent a repeat biopsy within 1–2 years of enrollment and 17.2% were found to have cancer of which 30% were high grade (Gleason score >7). At the repeat biopsy between years 3 and 4, 11.7% of 2343 patients were found to have cancer of which 21% were high grade. Considering only patients with primary Gleason pattern 4 or 5, 8.7% and 2.6% of the cancers were high grade at the 2- and 4-year biopsy, respectively. This study also compared the relative merit of PCA3 and F/T PSA in the repeat biopsy population. No significant difference was seen between these two markers for predicting cancer (Andriole et al, 2010).
The Gleason system is the most commonly employed grading system. The system relies on the low-power appearance of the glandular architecture under the microscope. In assigning a grade to a given tumor, pathologists assign a primary grade to the pattern of cancer that is most commonly observed and a secondary grade to the second most commonly observed pattern in the specimen. Grades range from 1 to 5 (Figure 23–3). If the entire specimen has only one pattern present, then both the primary and secondary grade are reported as the same grade (eg, 3 + 3). The Gleason score or Gleason sum is obtained by adding the primary and secondary grades together.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree