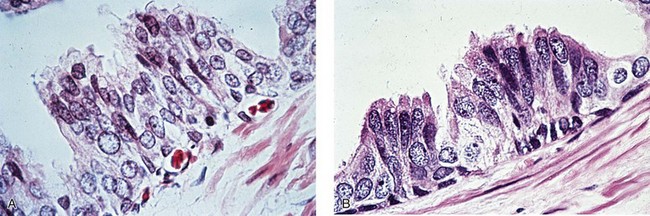
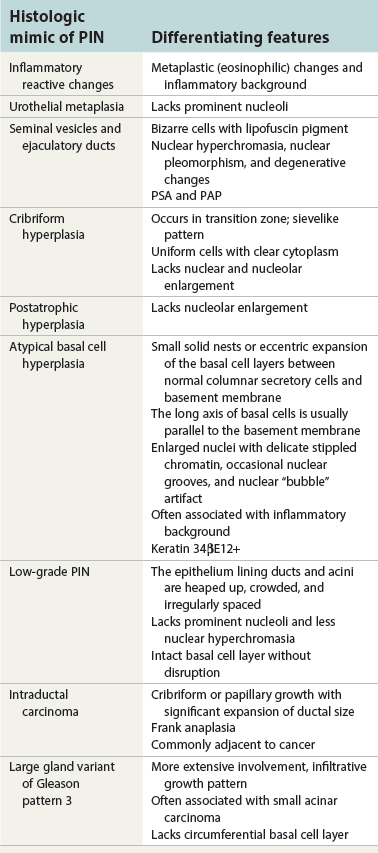
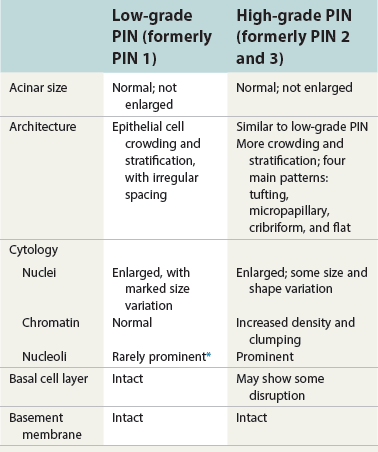
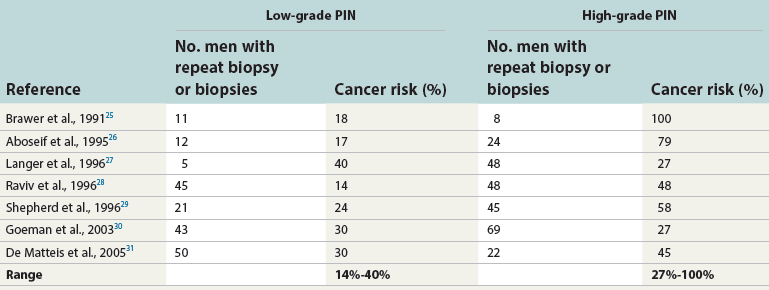
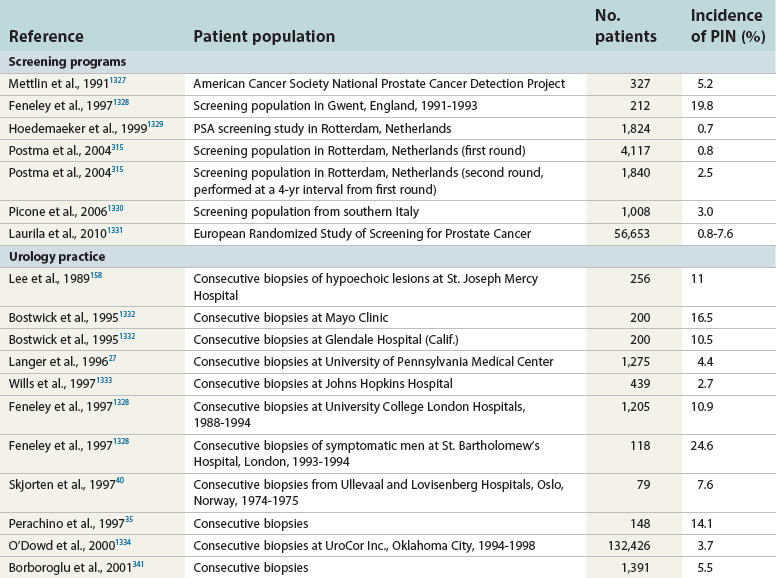
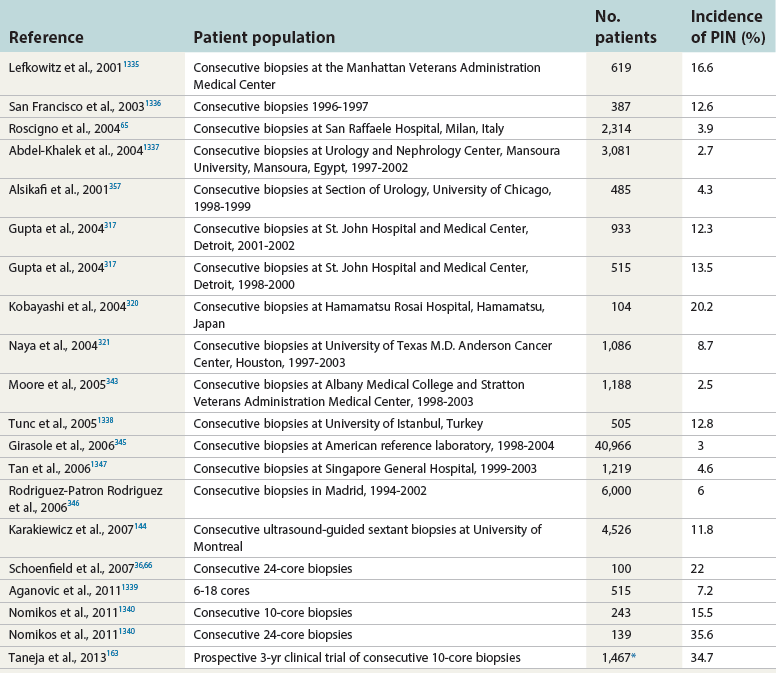
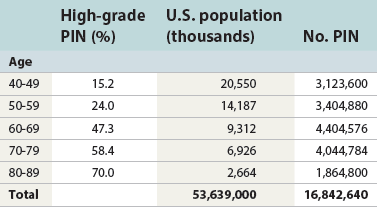
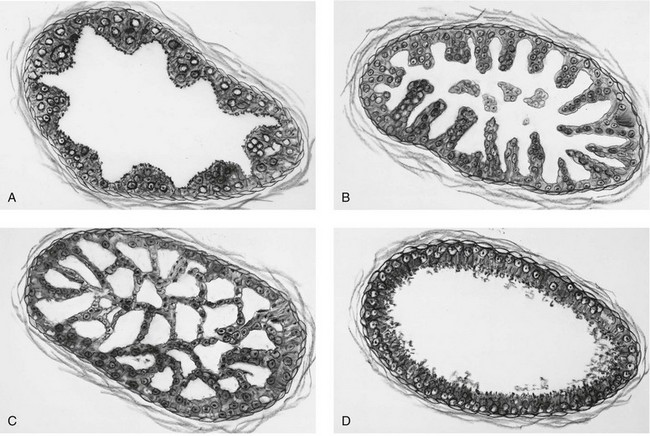
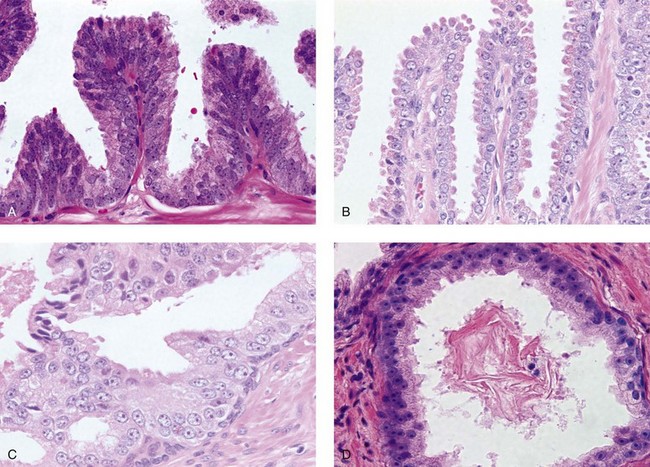
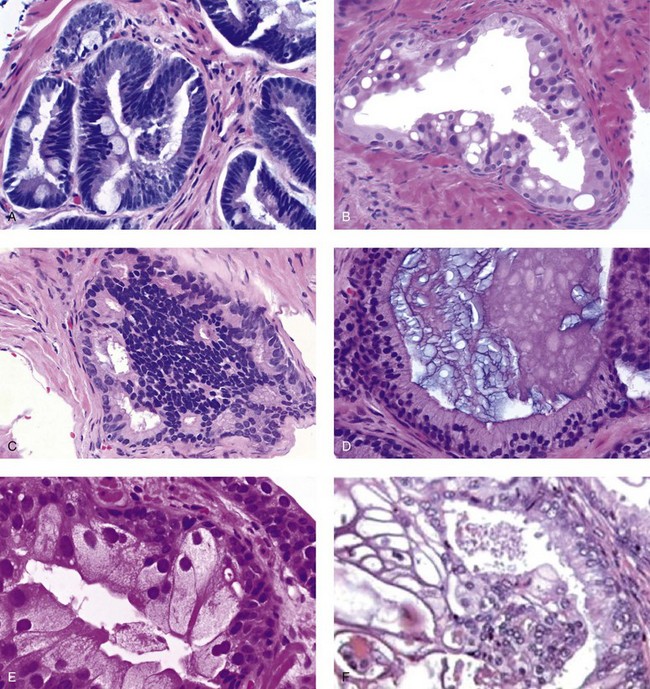
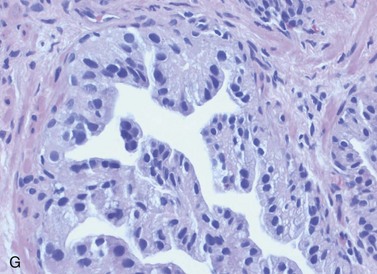
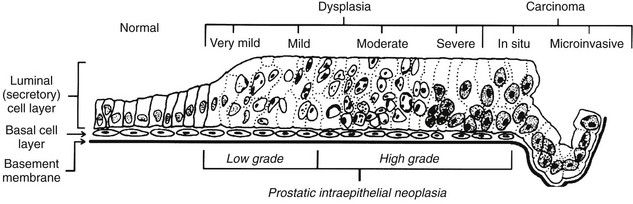
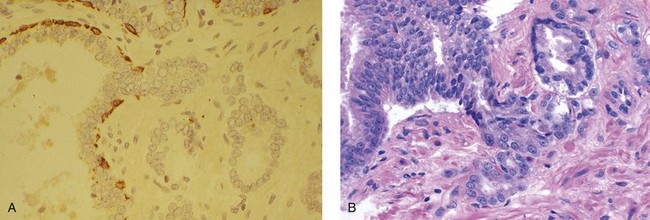
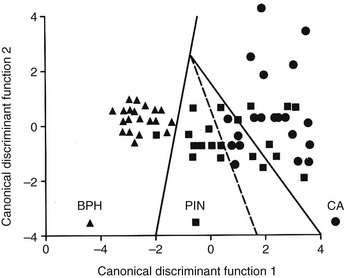
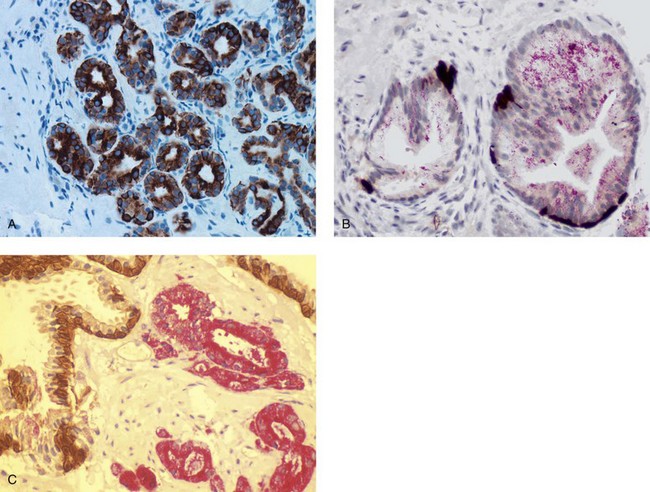
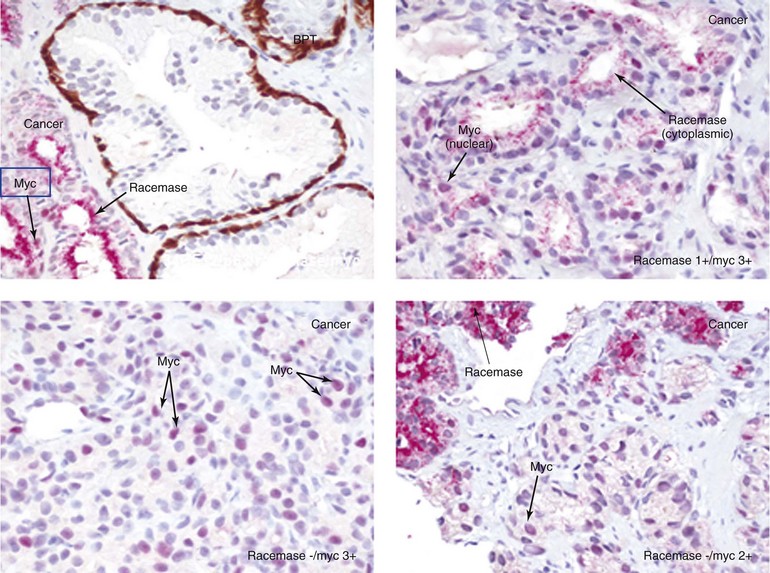
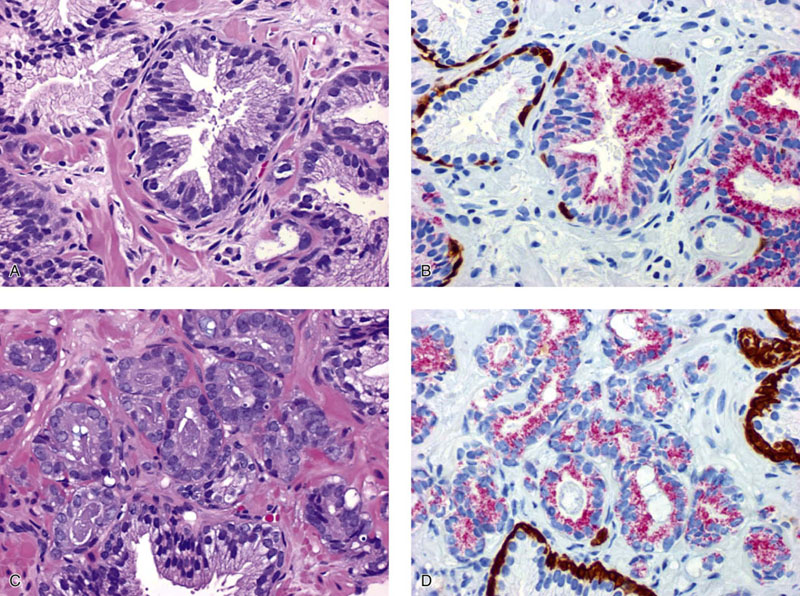
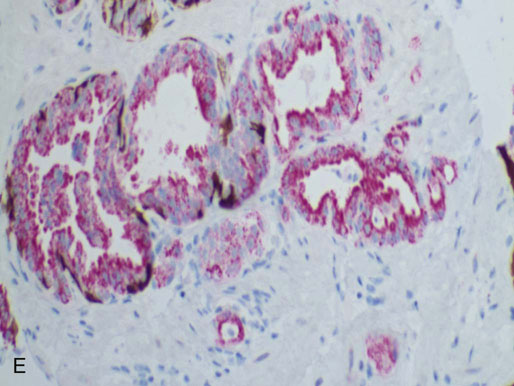
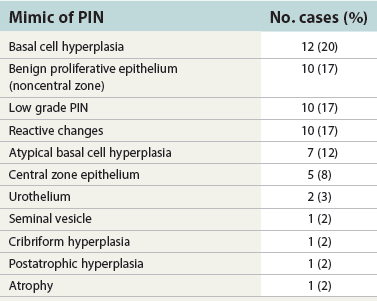
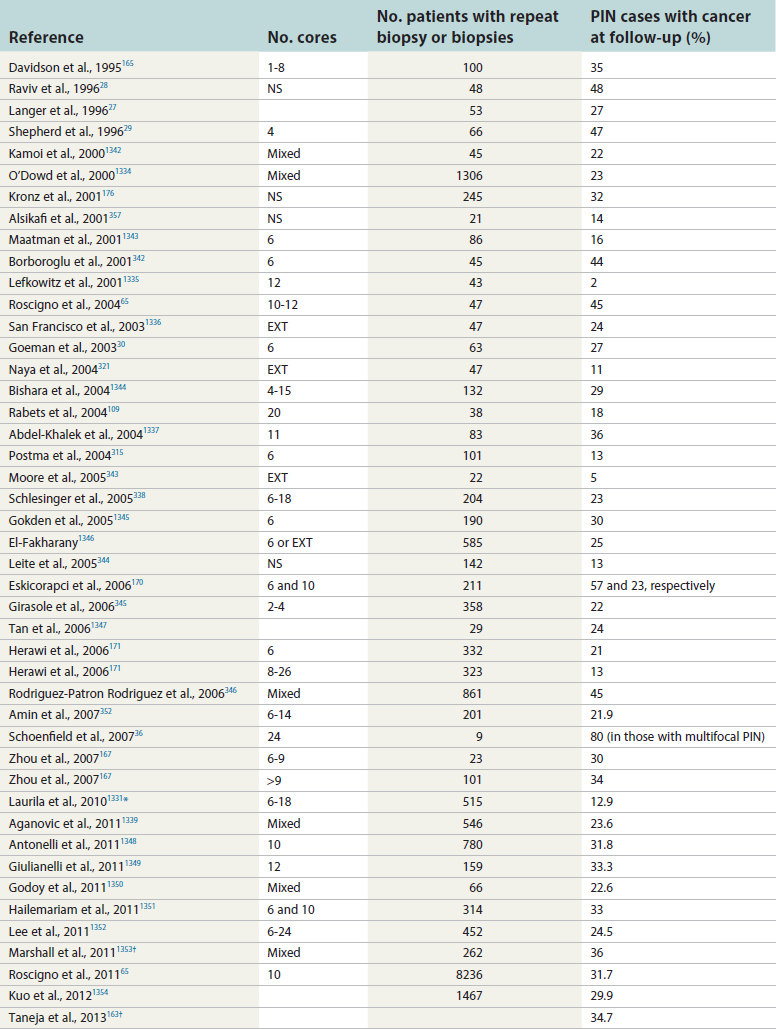
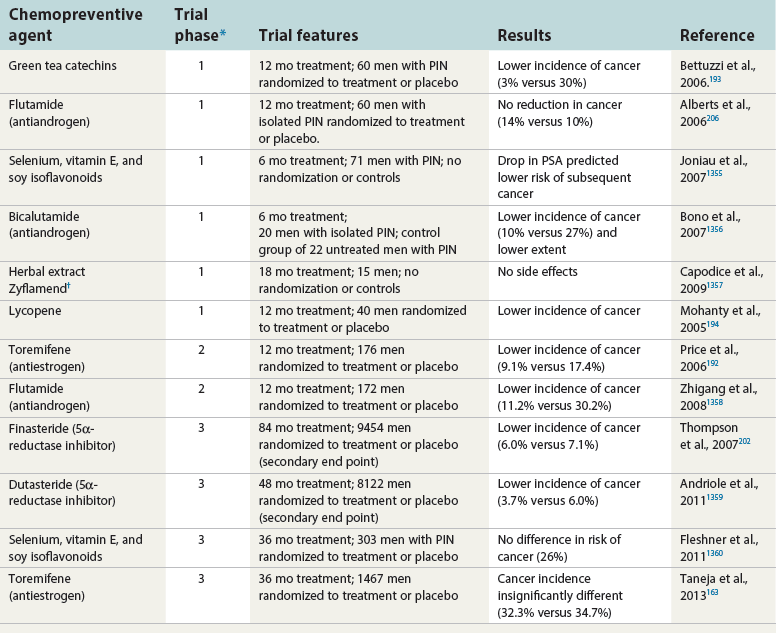
Chapter 9 Prostate cancer is the most common cancer in men in the United States and is third only to lung and colorectal cancer as a cause of cancer death. In 2012 an estimated 28,170 Americans died of prostate cancer and 241,780 new cases were diagnosed.1 The probability of developing clinically detected prostate cancer rose from 1 in 38 in men between 40 and 59 years of age to 1 in 15 for men between 60 and 69 years and 1 in 8 for men 70 and older; for all men, the overall probability was 1 in 6.1 Despite prevalence at autopsy of up to 80% by age 80 years,2 the clinical incidence is much lower, indicating that most men die with prostate carcinoma rather than of prostate carcinoma. Little is known about the causes of prostate cancer despite its high incidence and prevalence. This chapter reviews the pathology of adenocarcinoma of the prostate and other prostatic tumors. Issues of grading and staging are addressed, and diagnostic and prognostic markers are discussed. Prostatic intraepithelial neoplasia (PIN) refers to the preinvasive end of the continuum of cellular proliferations within the lining of prostatic ducts, ductules, and acini.3 High-grade PIN is the earliest accepted stage in carcinogenesis, possessing most of the phenotypic, biochemical, and genetic changes of cancer without invasion into the fibromuscular stroma.4,5 The World Health Organization (WHO) contends that PIN is the only demonstrated preinvasive lesion for prostate cancer.6 Other potential but unproved candidates for premalignancy in the prostate include atypical adenomatous hyperplasia (Chapter 8), malignancy-associated changes arising in normal-appearing epithelium,7–9 and atrophy (Chapter 8). Initial references to the lesion we now know as PIN apparently were made by early authors such as Kastendieck and Helpap,10 but they did not provide reproducible criteria and distinguish their findings from mimics of PIN. In 1965, McNeal11 emphasized the possible premalignant nature of proliferative changes in the prostatic epithelium, but his description included a variety of findings. More than 30 years later, McNeal and Bostwick12 described, for the first time, reproducible diagnostic criteria for the recognition of what they referred to as intraductal dysplasia, and introduced a three-grade classification system. The following year, Bostwick and Brawer13 proposed the term prostatic intraepithelial neoplasia as a replacement for intraductal dysplasia, and this new term was promulgated in 1989 at a workshop on prostate preneoplastic lesions sponsored by the American Cancer Society and National Cancer Institute.14 The diagnostic term prostatic intraepithelial neoplasia was subsequently endorsed at numerous multiple multidisciplinary and pathology consensus meetings.6,15–20 Terms such as intraductal dysplasia, severe dysplasia, large acinar atypical hyperplasia, and duct-acinar dysplasia were discouraged and have ceased to be used in routine practice.21,22 The term intraductal carcinoma was discouraged 20 years ago, but has seen a resurgence as a separate entity that can be mistaken for PIN (see later discussion). The 1989 conference also recommended compression of the PIN classification into two grades: low-grade (formerly PIN grade l) or high-grade PIN (formerly PIN grades 2 and 3) (Table 9-1).14 The clinical significance of high-grade PIN was considered substantial at that time, whereas low-grade PIN was considered largely inconsequential, a belief that has been reinforced in subsequent decades and persists today. Table 9-1 Diagnostic criteria for prostatic intraepithelial neoplasia PIN, Prostatic intraepithelial neoplasia. Interobserver agreement among pathologists for high-grade PIN is “good to excellent.”20,23,24 However, this is not true for low-grade PIN; also, it has a much lower predictive value for cancer that limits its clinical utility (Table 9-2),20,25–31 and most do not routinely report this finding today except in research studies.20,25–31 Thus the term prostatic intraepithelial neoplasia is now used interchangeably with high-grade prostatic intraepithelial neoplasia by most investigators. High-grade PIN is considered a standard diagnosis that must be included as part of the reported pathologic evaluation of biopsy specimens, transurethral resections, and radical prostatectomy specimens.20 The diagnostic utility when cancer is already present is unclear, but 69% of urologic pathologists still report PIN in this setting.20 Table 9-2 Comparative cancer risk in patients with low-grade versus high-grade prostatic intraepithelial neoplasia* PIN, Prostatic intraepithelial neoplasia. *Includes only those studies simultaneously reporting low-grade and high-grade PIN. The mean reported incidence of isolated high-grade PIN is 9% (range, 4% to 24%) of prostate biopsies (Table 9-3), similar to that in our personal experience in New York in 2012 (data not shown). Given that an estimated 1,300,000 prostate biopsies are performed annually, a reasonable estimate is that approximately 115,000 new cases of high-grade PIN without cancer32,33 are reported each year, with a prevalence of more than 16 million (Table 9-4). Table 9-3 Incidence of isolated high-grade prostatic intraepithelial neoplasia in prostatic needle biopsy samples PIN, Prostatic intraepithelial neoplasia. Table 9-4 Estimated prevalence of high-grade prostatic intraepithelial neoplasia in the United States The incidence of PIN varies according to the population of men under study (see Tables 9-3 and 9-4).34,35 The lowest likelihood is in men participating in prostate-specific antigen (PSA) screening and early detection studies, with an incidence of PIN ranging from 0.7% to 20%.34,35 Men seen by urologists in practice have PIN in 4.4% to 25% of biopsy samples. The relationship between the number of cores sampled and the incidence of PIN on needle biopsy is controversial, although most agree that greater sampling increases the yield of both PIN and cancer. The incidence of PIN on 24-core saturation biopsy was 22% according to one study36; another study showed an incidence of 45% by saturation biopsy.37 Those undergoing transurethral resection have the highest likelihood of PIN, varying from 2.8% to 33%.25,38–40 In such cases, all tissue should be examined, but serial sections of suspicious foci are usually not necessary. Unfortunately, needle biopsy specimens fail to show the suspicious focus on deeper levels in approximately half of cases, often precluding assessment by immunohistochemistry and compounding the diagnostic dilemma. The incidence and extent of PIN increase with patient age (see Table 9-4).41 An autopsy study of step-sectioned whole-mount prostates from older men showed that the prevalence of PIN in prostates with cancer increased with age, predating the onset of carcinoma by more than 5 years.16,42,43 A similar study revealed that PIN is first seen in men in their twenties and thirties (9% and 22% frequency, respectively) and precedes the onset of carcinoma by more than 10 years.42 Most foci of PIN in young men were low grade, with increasing frequency and volume of high-grade PIN with advancing age.44 Race and geographic location also appear to influence the incidence of PIN after controlling for patient age.6,44 For example, African-American men had a greater prevalence of PIN than Caucasians in the 50- to 60-year-old age group, the decade preceding detection of most prostate cancers.16,45 African-American men also had the highest incidence of cancer (~50% more than Caucasians).2,16,46,47 In contrast, Japanese men living in Osaka, Japan had a significantly lower incidence of PIN than those residing in the United States and Asians had the lowest clinically detected rate of prostate cancer.48,49 Interestingly, Japanese men diagnosed with PIN also had an increased likelihood of developing prostate cancer, indicating that PIN is also a precursor of clinical prostate cancer in Asian men.50 Differences in the frequency of PIN in the 50- to 60-year-old age group across races essentially mirror the rates of clinical prostate cancer observed in the 60- to 70-year-old age group.48,51 The likely causal association of PIN with prostatic adenocarcinoma is supported by the observation that the prevalence of both increase with patient age and that PIN precedes the onset of prostate cancer by less than 1 decade (Table 9-5).2,42,51,52 The severity and frequency of PIN in prostates with cancer are greatly increased (73% of 731 specimens) in contrast to prostates without cancer (32% of 876 specimens).41,53,54 When high-grade PIN was identified on sextant needle biopsy, a 50% risk was noted of finding carcinoma on subsequent biopsies within 3 years,55 although this risk was lower when more than six cores were obtained. This decline in predictive value is expected given the increased sampling for cancer with a greater number of core biopsies (see later discussion). PIN is characterized by cellular proliferation within preexisting ducts and acini, with cytologic changes mimicking cancer, including nuclear and nucleolar enlargement (Figs. 9-1 to 9-3).4,56 Inversion of the normal orientation of epithelial proliferation from the basal cell compartment to the luminal surface occurs, similar to that in adenomas in the colon.57,58 The requisite nucleolar enlargement must be present in at least 10% of cells within the focus to be considered diagnostic; however, overstaining, hyperchromasia, and nuclear overlap may obscure the nucleolar features. Overexpression of the MYC oncogene is responsible for the increased nucleolar number and size in PIN and cancer.59 Fig. 9-2 Architectural patterns of high-grade prostatic intraepithelial neoplasia. A, Tufting pattern. B, Micropapillary pattern. C, Cribriform pattern. D, Flat pattern. (From Bostwick DG, Amin MB, Dundore P, et al. Architectural patterns of high grade prostatic intraepithelial neoplasia. Hum Pathol 1993;24:298-310, with permission.) Fig. 9-3 Architectural patterns of high-grade PIN. Compare with artist’s renditions in Figure 9-2. A, Tufting and early micropapillary patterns. B, Micropapillary pattern. C, Cribriform pattern. D, Flat pattern. High-grade PIN appears in four main patterns: tufting, micropapillary, cribriform, and flat (see Figs. 9-1 to 9-3).60 The tufting pattern is the most common, present in 97% of cases, although most cases have multiple patterns. No clinically important differences between the architectural patterns have been identified, and their recognition appears to be only of diagnostic utility. Sporadic retrospective reports have suggested that the cribriform or micropapillary patterns may indicate higher risk for coexistent cancer, but this has been repeatedly refuted. Other unusual patterns of PIN include the signet ring cell pattern, small cell neuroendocrine (oat cell) pattern, mucinous pattern, microvacuolated (foamy-gland) pattern, inverted (hobnail) pattern,61 and PIN with squamous differentiation (Table 9-6 and Fig. 9-4).62,63 Fig. 9-4 Variants of high-grade PIN. A and B, Signet ring cell. C, Small cell neuroendocrine. D, Mucinous. E, Foamy gland. F, Squamous. G, Inverted. The presence of extensive PIN appears to be more predictive of cancer than the more common isolated single acinus with PIN (see later discussion).64–66 The presence of intraluminal crystalloids in combination with PIN is a more compelling indication for repeat biopsy than PIN alone.67 PIN spreads through prostatic ducts in multiple different patterns, similar to carcinoma. In the first pattern, neoplastic cells replace the normal luminal secretory epithelium, with preservation of the basal cell layer and basement membrane. This pattern often has a cribriform or near-solid appearance. Foci of high-grade PIN may be difficult to distinguish from intraductal or intraacinar spread of carcinoma by routine light microscopy (see later discussion).68 In the second pattern, direct invasion occurs through the ductal or acinar wall, with disruption of the basal cell layer. In the third pattern, neoplastic cells invaginate between the basal cell layer and columnar secretory cell layer (pagetoid spread), a very rare finding. The proliferative activity, defined as Ki67 labeling index, was lower in PIN (mean, 6%; range, 2% to 15%) than in ductal adenocarcinoma; the combination of histologic features and measurements of cellular proliferation help distinguish these findings in limited tissue samples.69,70 By electron microscopy, high-grade PIN displayed features that were intermediate between those of benign epithelium and adenocarcinoma.71 These included the presence of cells with a variable number of cytoplasmic secretory vacuoles, luminal apocrine blebs, large nuclei with coarsely clumped chromatin, enlarged nucleoli, prominent apical microvilli, intact or discontinuous basal cell layer, and intact basement membrane. Occasional acini had luminal cells abutting the basement membrane without interposition of basal cells, and other acini with extremely attenuated basal cell cytoplasmic processes contained sparse bundles of intermediate filaments. Early stromal invasion, the earliest evidence of carcinoma, occurs at sites of acinar outpouching and basal cell disruption in acini with high-grade PIN (Figs. 9-5 and 9-6). Such microinvasion is present in 2% of high-power microscopic fields of PIN and is seen with equal frequency in all architectural patterns.18,60 At the transition from histologically normal epithelium to PIN, a surge in phosphorylated Akt (prosurvival protein) and a concomitant suppression of downstream apoptosis pathways (antisurvival proteins) occur that precede the transition to invasive cancer.72 Fig. 9-5 Morphologic continuum from normal prostatic epithelium through increasing grades of prostatic intraepithelial neoplasia to early invasive carcinoma, according to the disease-continuum concept. Low-grade prostatic intraepithelial neoplasia (grade 1) corresponds to very mild–to-mild dysplasia. High-grade prostatic intraepithelial neoplasia (grades 2 and 3) corresponds to moderate-to-severe dysplasia and carcinoma in situ. The precursor state ends when malignant cells invade the stroma; this invasion occurs where the basal cell layer is disrupted. Dysplastic changes occur in the superficial (luminal) secretory cell layer, perhaps in response to luminal carcinogens. Disruption of the basal cell layer accompanies the architectural and cytologic features of high-grade prostatic intraepithelial neoplasia and appears to be a necessary prerequisite for stromal invasion. Basement membrane is retained with high-grade prostatic intraepithelial neoplasia and early invasive carcinoma. (Modified from Bostwick DG, Brawer MK. Prostatic intra-epithelial neoplasia and early invasion in prostate cancer. Cancer 1987;59:788-794, with permission.) Fig. 9-6 Prostatic intraepithelial neoplasia (PIN) and microinvasion. A, Basal cell layer disruption in high-grade PIN (left) and absent basal cell layer in cancer (right). The tongue of cells (center) protruding from the large acinar structure with PIN is thought to represent early invasion (basal cell–specific antikeratin 34βE12 immunostain). B, Another case of PIN with invagination suspicious for early invasion. The mean volume of PIN in prostates with cancer is 1.2 to 1.32 cc, and the volume increases with increasing pathologic stage, Gleason grade, positive surgical margins, and perineural invasion.41,73 These findings underscore the close spatial and biologic relationship of PIN and cancer and may result from an increase in PIN with increasing cancer volume. PIN and cancer are usually multicentric.5,41,60,65 PIN is multicentric in 72% of radical prostatectomies with cancer, including 63% of those involving the nontransition zone and 7% of those involving the transition zone; 2% of cases have concomitant single foci in all zones.41 The peripheral zone of the prostate, the area in which the majority of cases of prostate cancer occur (70% or more), is also the most common location for PIN.41,42,51,60,74 Cancer and PIN are frequently multicentric in the peripheral zone, indicating a “field” effect similar to urothelial carcinoma of the bladder. Central zone cancer is more likely to be associated with PIN in the central zone than the peripheral zone.75 Despite common multifocality, PIN still may be a monoclonal process. High-grade PIN and prostate cancer are morphometrically and phenotypically similar (Fig. 9-7). PIN occurs primarily in the peripheral zone and is seen in areas in continuity with prostate cancer.6,17,41,74,76–78 PIN and prostate cancer are multifocal and heterogeneous.41,79,80 Increasing rates of aneuploidy and angiogenesis as the grade of PIN progresses are further evidence that high-grade PIN is precancerous.77,81–83 Prostate cancer and high-grade PIN have similar proliferative and apoptotic indices.48,84–87 Swedish investigators note that PIN cannot be diagnosed by fine needle aspiration alone,88 although this has been refuted.89 The diagnosis of PIN in combination with atypical small acinar proliferation suspicious for but not diagnostic of malignancy (ASAP) is discussed later. Fig. 9-7 Scatterplot of the spatial distribution of benign prostatic hyperplasia (BPH), prostatic intraepithelial neoplasia (PIN), and cancer (CA). The cases appear as continuous categories, with overlap mainly between PIN and cancer. The two lines divide the scatterplot into three parts, corresponding to three categories. The part corresponding to PIN is subdivided into two parts (interrupted line), separating low-grade PIN (close to BPH) and high-grade PIN (close to cancer). (Modified from Montironi R, Scarpelli M, Sisti S, et al: Quantitative analysis of prostatic intra-epithelial neoplasia on tissue sections. Anal Quant Cytol Histol 1990;12:366-372, with permission.) We routinely employ a battery of four immunostains that, in combination, are invaluable in separating benign and malignant conditions of the prostate (Table 9-7). Select antibodies such as antikeratin 34βE12 (high-molecular-weight keratin) and p63 (see later discussion) are used to stain tissue sections for the presence of basal cells,90,91 recognizing that PIN retains an intact or fragmented basal cell layer but cancer does not (Fig. 9-8). In addition, racemase and c-myc are useful for staining of the dysplastic secretory cells of PIN (see later discussion). Table 9-7 Routine four stains in prostate cancer diagnosis* *Bostwick Laboratories, Glen Allen, Va, and multiple other laboratories. Fig. 9-8 Immunostain with keratin 34βE12 and racemase. A, Intact basal cell layer in atrophic acini. B, Basal cell layer is disrupted in high-grade PIN; note positive racemase staining (red). C, Absent basal cell layer in cancer; note positive racemase staining (red). We routinely generate unstained intervening sections of all prostate biopsy samples for possible future immunohistochemical staining, recognizing that small foci of concern are often lost when the tissue block is recut; one study reported loss of the suspicious focus in 31 of 52 cases.92 Monoclonal basal cell–specific antikeratin 34βE12 stains the cytoplasm of most normal basal cells of the prostate, with continuous intact circumferential staining in many instances. No staining occurs in secretory and stromal cells. This marker is the most commonly used immunostain for prostatic basal cells,93,94 and methods of use with paraffin-embedded sections have been optimized.95 Keratin 34βE12 is formalin sensitive and requires pretreatment by enzymes or heat if formalin-based fixatives are used. After pepsin predigestion or microwaving, progressive loss of immunoreactivity is seen from 1 week or longer of formalin fixation. Heat-induced epitope retrieval with a hot plate yielded consistent results with no decrease in immunoreactivity and as long as 1 month of formalin fixation.95 The staining intensity was consistently stronger at all periods of formalin fixation when the hot plate method was used, in contrast to pepsin predigestion or microwaving. Weak immunoreactivity was rarely observed in cancer cells after hot plate treatment, but not with pepsin predigestion or microwave antigen retrieval. Steam–ethylenediaminetetraacetic acid in combination with protease significantly enhanced basal cell immunoreactivity in contrast to protease treatment alone in benign prostatic epithelium.96 Nonreactive benign acini were always the most peripheral acini in a lobule, a small cluster of outpouched acini furthest from a large duct, or the terminal end of a large duct.97 More proximal acini had a discontinuous pattern of immunoreactivity. Increasing grades of PIN are associated with progressive disruption of the basal cell layer, according to studies using antikeratin 34βE12. Basal cell layer disruption is present in 56% of cases of high-grade PIN and is more frequent in acini adjacent to invasive carcinoma than in distant acini. Early invasive carcinoma occurs at sites of glandular outpouching and basal cell discontinuity in association with PIN.13 The cribriform pattern of PIN may be mistaken for the cribriform pattern of ductal adenocarcinoma, and the use of antikeratin staining is often useful in making this distinction, although exceptions occur (see later discussion).98 Cancer cells consistently fail to react with this antibody, although admixed benign acini may be misinterpreted as cancerous staining. Thus, immunohistochemical stains for antikeratin 34βE12 may show the presence or absence of basal cells in a small focus of atypical glands, helping to establish a benign or malignant diagnosis, respectively. We think this antibody can be employed successfully if the results in combination with the light microscopic findings are judiciously interpreted; relying solely on the absence of immunoreactivity (absence of basal cell staining) to render the diagnosis of cancer is without precedent in diagnostic immunohistochemistry and is discouraged.99 Nonetheless, some studies have noted that the rate of equivocal cases can be reduced considerably,100 by 68%,93 or from 5.1% to 1.0%101 by addition of this immunohistochemical marker. Evaluation of prostate biopsy samples after therapy such as radiation therapy may be one of the most useful roles for antikeratin 34βE12 (see later discussion).58 In addition to PIN and cancer, basal cell layer disruption or loss also occurs in inflamed acini, atypical adenomatous hyperplasia, and atrophy and postatrophic hyperplasia and may be misinterpreted as cancer if one relies exclusively on the immunohistochemical profile of a suspicious focus. Furthermore, basal cells of Cowper glands may not express keratin 34βE12,102 although this has been disputed.103 Rare (0.2%) cases of adenocarcinoma have been reported that focally or weakly express keratin 34βE12, including foci of metastatic high-grade adenocarcinoma.104 Basal cell hyperplasia is a histologic mimic of cancer, and use of antikeratin 34βE12 is recommended in any equivocal cases that include this lesion in the differential considerations because it is invariably positive in that lesion.105,106 p63 is a nuclear protein that is at least as sensitive and specific for the identification of basal cells in diagnostic prostate specimens as high-molecular-weight cytokeratin staining.90,107–116 One report found that p63 was more sensitive than keratin 34βE12 in staining benign basal cells, particularly in specimens from transurethral resection of the prostate (TURP), offering a slight advantage in diagnostically challenging cases.117 Another study demonstrated basal cell cocktail (34βE12 and p63) increased the sensitivity of the basal cell detection and reduced staining variability, thus rendering basal cell immunostaining more consistent.90 Triple staining with racemase, high-molecular-weight cytokeratin, and p63 is commonly used for the diagnosis of prostate cancer (Figs. 9-9 and 9-10; see Fig. 9-8), although we think that the addition of c-myc (quadruple stain) optimizes diagnostic yield (see later discussion).108,110,113,114,118 Fig. 9-9 Quadruple-antibody cocktail (keratin 34βE12/p63/racemase/c-myc) staining in benign prostatic epithelium (top left panel) and cancer (other three panels). BPT, Benign prostatic tissue. Fig. 9-10 Comparison of hematoxylin and eosin (H&E) and triple-antibody cocktail (keratin 34βE12/p63/racemase) staining. A, H&E of high-grade prostatic intraepithelial neoplasia (HGPIN) (large central acinus) with adjacent benign acini (left) in a biopsy. B, In contrast to A, immunostain of HGPIN reveals both red cytoplasmic granular staining pattern of racemase and dark brown cytoplasmic (34βE12) and nuclear (p63) staining of basal cells. C, H&E of adenocarcinoma (Gleason 3 + 3 = 6) on biopsy with adjacent benign acini. D, In contrast to C, the cancerous glands show epithelial cells with only red cytoplasmic granular staining pattern of racemase, whereas adjacent benign glands show basal cells with only dark brown cytoplasmic (34βE12) and nuclear (p63) staining. E, PIN (left side) is bounded by basal cells, whereas cancer acini that are budding off (right) do not display basal cells. Aberrant p63 staining is occasionally observed in primary and metastatic prostate cancer, so diagnosis should not rely exclusively on this marker.119 Maintenance of normal stem cell or reserve cell populations in prostatic epithelium is regulated by p63, and alteration of p63 expression is considered to have an oncogenic role in prostate cancer; expression of cytoplasmic aberrance of p63 is associated with high ALDH1A1 expression.120 Cytokeratin 5 (CK5) and CK14 mRNA and protein are expressed in the basal cells of benign acini and PIN, and CK14 mRNA is present in low levels in the luminal cells of some foci of PIN; thus, if PIN is derived from basal cells, as is currently thought, CK14 translation is depressed and a low level of CK14 mRNA may persist.121 CK8 mRNA and protein were constitutively expressed in all epithelia of the normal and neoplastic prostate. CK19 mRNA and protein were expressed in both basal and luminal cells of benign acini. CK16 mRNA was expressed in a similar pattern as CK19, but CK16 protein was not detected.121 Other markers of basal cells include proliferation markers, differentiation markers, and genetic markers. The preferential localization of many of these markers in basal cells but not in secretory cells suggests that they play a role in growth regulation. p63 is a nuclear marker that may be useful for separating PIN and cancer from benign mimic. Basal cells display immunoreactivity at least focally for keratins 5, 10, 11, 13, 14, 16, and 19; of these, only keratin 19 is also found in secretory cells.25,122 Keratins found exclusively in the secretory cells include 7, 8, and 18. Basal cells usually do not display immunoreactivity for PSA, prostatic acid phosphatase (PAP), and S100 protein, and only rare single cells stain with chromogranin and neuron-specific enolase (NSE). Conversely, the normal secretory luminal cells invariably stain with PSA and PAP. Prostatic basal cells do not usually display myoepithelial differentiation,123,124 in contrast to basal cells in the breast, salivary glands, pancreas, and other sites. Racemase (i.e., α-methylacyl–coenzyme A racemase [AMACR, or P504S]) also has proved useful for separating benign and neoplastic acini, including evaluation of PIN, ASAP, and separation of cancer from hormonally treated benign acini. This well-characterized enzyme catalyzes the conversion of several (2R)-methyl branched-chain fatty acyl-CoAs to their (S)-stereoisomers. Analysis of mRNA levels of racemase revealed an average upregulation of ninefold in prostate cancer. The gene for AMACR is greatly overexpressed in prostate cancer cells.125 Its advantage over antikeratin 34βE12 is its positive granular cytoplasmic staining in cancer cells, with little or no staining in benign acini. In PIN, monoclonal and polyclonal antibodies to racemase were positive in 77% and 91% of foci, respectively,126 consistent with findings in other studies (see Fig. 9-10).125,127–130 Because racemase is not specific for prostate cancer and is present in high-grade PIN (>90%) and nephrogenic adenoma, this staining must be interpreted with caution and the diagnosis of PIN or prostate cancer should be rendered only with convincing histologic evidence.131 Moderate to strong racemase expression in PIN is indicative of an associated adenocarcinoma.132 Racemase is negative in up to 20% of cancer cases. TMPRSS2:ERG gene rearrangement was found to be highly specific for and present in approximately 50% of prostate cancers and 29% of foci of PIN, according to immunohistochemical staining with anti-ERG antibody that was highly correlated with TMPRSS2:ERG gene rearrangement status.133–138 ERG rearrangements are associated with loss of the tumor suppressor gene PTEN, and this cooperation promotes progression of PIN to invasive cancer.139 In a prospective study of ERG staining of biopsy specimens with PIN, Gao and colleagues140 found that this marker was highly predictive of cancer on repeat biopsy (95% in positive cases versus 5% in negative cases). Conversely, He and associates141 found 38% risk for cancer on repeat biopsy that was independent of ERG-staining status with PIN. Immunohistochemical expression of ERG in PIN was strongly predictive of ERG status in coexistent prostate cancer, with more than 95% concordance.142 The histologic differential diagnosis of PIN includes a variety of benign conditions, including atrophy, postatrophic hyperplasia, atypical basal cell hyperplasia, cribriform hyperplasia, and metaplastic changes associated with radiation, infarction, and prostatitis (Tables 9-8 and 9-9) (Chapter 8).142a Many of these display mild architectural and cytologic atypia, including enlarged nucleoli. The diagnostic difficulty is compounded when these findings appear in small, cauterized, or distorted specimens. Biopsy specimens submitted with incomplete patient history should be interpreted with caution. Table 9-9 Mimics of high-grade prostatic intraepithelial neoplasia: overdiagnosis of prostatic intraepithelial neoplasia in 60 consecutive cases142a Neoplastic mimics of PIN include intraductal carcinoma (see later discussion), cribriform adenocarcinoma, ductal (endometrioid) carcinoma, and urothelial carcinoma. Proliferative activity, defined as Ki67 labeling index, was higher in ductal carcinoma than in PIN (33% versus 6%).69,70 Stratified epithelium in noncribriform glands of prostate cancer can also resemble high-grade PIN. Recognition of this fact and immunohistochemical evaluation of stratified glands may be indicated to correctly diagnose those glands as prostate cancer.143 Inflammation has been proposed as a causative mechanism for prostatic carcinogenesis, but the evidence to date is inconclusive or contradictory, probably because of the ubiquitous presence of inflammation in the prostate (Chapter 8). In a 2012 study of needle biopsy samples, chronic inflammation was identified in 7.7% and conferred a protective effect from PIN and cancer even after adjusting for age, digital rectal examination (DRE) findings, serum PSA, and prostate volume.144 Other reports found that inflammation was inversely related to cancer risk,145 atrophy was marginally related, and PIN was a significant factor.146 Proliferative changes of the epithelium are linked to carcinogenesis in almost all epithelial malignancies. Proliferative regenerative change, referred to in the prostate as proliferative inflammatory atrophy, has been postulated as a premalignant lesion. The hypothesis is that cellular injury and regeneration characteristic of inflammatory atrophy is induced by inflammation and release of reactive oxygen species (oxidative stress) resulting from insult caused by chemicals (e.g., dietary carcinogens), physical factors (e.g., arteriosclerosis-induced ischemia), or bacteria. The regenerating cells are at increased risk for mutation, which in turn predisposes them to cancer initiation, promotion, and progression. The clinical implication of this hypothesis is that antiinflammatory drugs could potentially block procarcinogenic inflammatory processes.147 Evidence to date is inconclusive as to whether atrophy is a precursor of PIN, direct precursor of cancer that bypasses PIN, or simply an epiphenomenon (see Chapter 8). What is clear is that any association with cancer requires proliferative changes, and these changes in the setting of atrophy are most often associated with inflammation. Inflammation is often linked with infectious and noninfectious prostatitis, and emerging data indicate a causative role for inflammation in prostate cancer development over time.148,149 Despite the controversy regarding the role of atrophy (or lack thereof), virtually all authors agree that inflammation-induced oxidative stress is the most plausible explanation for initiation of prostatic carcinogenesis. Biopsy remains the definitive method for detecting PIN and early invasive cancer. Serum PSA concentration may be elevated in some patients with PIN,150 but these results have been refuted in most published reports.151–153 Poor correlation of PIN and PSA density has been noted according to studies of radical prostatectomy specimens and preoperative serum concentration.152 Mean PSA increased from 8.4 to 11.6 ng/mL in patients with PIN who developed cancer within 2 years; those with PIN who did not develop cancer during this interval had an increase in PSA from 4.8 to 5.9 ng/mL or decrease from 5.1 to 4.6 ng/mL. These findings were confirmed in a 2007 screening study in which the median PSA velocity was significantly greater in men with PIN who were subsequently diagnosed with cancer.154 A velocity threshold of 0.75 ng/mL/yr predicted which men with high-grade PIN would ultimately be diagnosed with cancer, and velocity was the only significant predictor of subsequent cancer detection on multivariate analysis.154 The ratio of free to total PSA is the same for patients with high-grade PIN and cancer, unlike low-grade PIN and hyperplasia.150,153,155,156 Many patients in these studies were later found to have cancer, so the elevation in serum PSA concentration and its derivatives may have resulted from undetected cancer. Mean insulin-like growth factor-I (IGF-I) and IGF-3 concentrations in men with PIN (130.2 ng/mL and 2394 ng/mL, respectively) were significantly higher than in controls (118.8 ng/mL and 2276 ng/mL, respectively), but these assays are not routinely available.157 By transrectal ultrasound, PIN may be hypoechoic, similar to carcinoma, although these findings have not been confirmed.158,159 Today, most urologists and radiologists do not believe that PIN is detectable by transrectal ultrasound because PIN is a microscopic finding that is below the detection threshold for this form of imaging. Likewise, magnetic resonance imaging (MRI) failed to accurately identify PIN.160 Positron emission tomography and computed tomography scans were unable to accurately separate PIN from other prostatic findings.161 The clinical importance of recognizing PIN is based on its strong association with prostatic carcinoma. PIN has a high predictive value as a marker for adenocarcinoma, so its identification in biopsy specimens warrants further search for concurrent or subsequent invasive carcinoma, especially when multifocal (Table 9-10).162 Results from the largest prospective randomized trial of its kind with 1467 untreated men with isolated PIN and central pathology revealed a cancer yield of 34.7% in repeat biopsies performed from 6 months to 36 months, underscoring the significance of this microscopic finding.163 This may be especially true for African-American men regardless of PSA level, according to a 2012 study (83% versus 7% incidence of cancer on repeat biopsy in men with and without PIN, respectively).164 If all procedures fail to identify coexistent carcinoma, close surveillance and follow-up are indicated. Table 9-10 Cancer risk in patients with high-grade prostatic intraepithelial neoplasia according to number of sampled needle biopsy cores EXT, extended; NS, not stated; PIN, prostatic intraepithelial neoplasia. †Prospective randomized trial (placebo arm of a prevention trial). The presence of isolated PIN in a set of sextant needle biopsies carries a risk ratio of 14.9 for cancer; this level of risk is considerably lower in men undergoing biopsies with a greater number of cores owing to greater sampling. PIN is a stronger predictor for subsequent cancer than the independent predictors of patient age (>65 years versus ≤65 years of age) and serum PSA concentration (>4 ng/mL versus ≤4 ng/mL); for these, the respective risk ratios are 3.5 and 3.64.165 PIN coexists with cancer in more than 85% of cases, according to studies employing whole-mounted totally embedded prostates. In one report, the likelihood of finding cancer increased with the biopsy time interval. The investigators reported a 32% incidence of cancer on repeat biopsy performed within 1 year, in contrast to a 38% incidence in biopsies obtained after 1 year.165 Other series also have found a high predictive value of PIN for cancer,166 although reports based on obtaining a greater number of cores show a lower predictive value (see Table 9-10).27,109,165,167 Follow-up biopsy within 1 year is suggested for patients with PIN if the serum PSA rises or in the presence of other clinical suspicion for cancer (Table 9-11).165,168 Some urologists perform “saturation” biopsies, consisting of more than 16 biopsies in one session, often with brief general anesthesia in the operating theater, in an effort to definitively exclude cancer.169 Most authors agree that identification of PIN in the prostate should not influence or dictate therapeutic decisions.168 We are aware of 21 radical prostatectomies that were purposely (3 cases) or inadvertently (18 cases) performed in patients whose biopsy samples contained only high-grade PIN; all but two of the cases contained adenocarcinoma in the surgical specimen (DG Bostwick, personal communication, 2007). Table 9-11 Suggested clinical responses to the diagnosis of prostatic intraepithelial neoplasia, atypical small acinar proliferation, and intraductal carcinoma on biopsy* *This assumes contemporary biopsies with ≥10 or more cores. Other clinical and laboratory factors such as serum PSA must always be considered. †Monitoring serum PSA is recommended in all cases. ‡Intraductal carcinoma almost always coexists with Gleason pattern 4 and 5 cancer. Multiple factors account for the decline in the predictive accuracy of high-grade PIN for cancer. The main factor is use of extended biopsy techniques that result in more thorough prostate sampling and higher cancer detection rates; thus the pool of patients with an isolated diagnosis of PIN is smaller.170 Another factor is the lower detection rate for, and difficulty in the detection of, the remaining small cancers; larger, clinically significant tumors also may escape detection. These factors lead to a higher frequency of negative repeat biopsy results and may reflect a new steady state and a new low plateau in the predictive accuracy of this marker. In one report, the investigators demonstrated that with six-core biopsies for both the initial and repeat biopsies, the risk for cancer after PIN was 14.1% in contrast to 31.9% in the group that had eight cores or more on follow-up with an initial six-core biopsy. The risk for cancer on biopsy within 1 year after a diagnosis of PIN (13.3%) was relatively low if good sampling (eight or more cores) was initially performed.171 The extent of PIN in needle biopsy specimens is another strong predictor of cancer on subsequent biopsy.36,64,66 The positive predictive value of PIN was 64%, with a sensitivity of 28% and specificity of 81%. For those with minute foci of PIN (isolated PIN that involves only one core) identified on an extended core biopsy, repeat biopsy may not be necessary within the first year in the absence of other clinical indicators of cancer such as elevated PSA.172,173 Cancer detection was significantly greater in patients with multifocal high-grade PIN than in those with unifocal PIN (70% versus 10%, respectively).36,65 When four or more cores contain PIN, the predictive value for cancer on subsequent biopsy was 39%.174,175 Kronz and co-workers176 found that the number of core samples with PIN was the only independent histologic predictor of a cancer diagnosis; risk for cancer was 30.2% with one or two cores with PIN, 40% with three cores, and 75% with more than three cores. After recutting of blocks with high-grade PIN, Rapp and colleagues177 found that 12.5% of patients had prostatic adenocarcinoma not previously detected. High-grade PIN in transurethral resection specimens is also an important predictive factor for prostate cancer.39,178 Among 14 patients with PIN and benign prostatic hyperplasia (BPH) followed for up to 7 years (mean, 5.9 years), 3 (21.4%) developed prostatic cancer.39 Mean serum PSA concentration was higher than in those who did not develop cancer (8.1 versus 4.6 ng/mL, respectively). All subsequent cancers apparently arose in the peripheral zone and were detected by needle biopsy. Thus all tissue should be submitted by the pathologist for examination when high-grade PIN is found in TURP specimens. The high predictive value of PIN for the development of subsequent cancer warrants reporting the presence of PIN in TURP specimens, according to the Cancer Committee of the College of American Pathologists. Conversely, PIN in the transition zone and central zone from Norwegian men was not predictive of subsequent cancer development.179 Short telomere length in PIN and the surrounding stroma was associated with an increased risk for cancer.180 Telomere length was also predictive of time from the original biopsy to diagnosis of cancer.181 Overexpression of p4EBP1 predicted cancer with sensitivity and specificity of 63% and 100%, respectively.182 Antonio and colleagues183 reported that patients with the metabolic syndrome (defined according to the National Cholesterol Education Program’s Adult Treatment Panel III criteria) were more likely to have widespread PIN (involving four or more cores) than isolated PIN (75% versus 25%) and more likely to have cancer on repeat biopsy (57%). Widespread PIN requires repeat biopsy, probably within 3 to 6 months. Isolated PIN probably does not require immediate repeat biopsy unless the serum PSA increases. However, a 2012 survey of German urologists found that the majority undertake repeat biopsy regardless of extent of PIN.184 Currently, treatment is not offered to patients who have high-grade PIN. Prophylactic radical prostatectomy or radiation is not an acceptable treatment for patients who have PIN only.185 The development and identification of acceptable agents to treat PIN would fill a therapeutic void. As noted earlier, androgen deprivation therapy and radiation therapy induce acinar atrophy and apoptosis that result in regression of high-grade PIN.15,185–190 Chronic therapy, however, would most likely be required to prevent new foci of PIN from invading and becoming clinical prostate cancer. Although more toxicity is likely to be tolerated for the treatment agents targeted to regress or inhibit PIN, in contrast to treating healthy patients to reduce prostate cancer incidence, androgen deprivation therapy has too many adverse effects in men to be clinically useful. New agents with better safety and lower side effect profiles are greatly needed because patients may be taking the agent at least until they attain 70 years of age (Table 9-12).183 Table 9-12 Chemoprevention clinical trials of men with prostatic intraepithelial neoplasia PIN, Prostatic intraepithelial neoplasia; PSA, prostate-specific antigen. *Trial phase is estimated because most of these trials were not U.S. Food and Drug Administration registration trials. †Extract consists of holy basil, green tea, ginger, Baikal skullcap, rosemary, Chinese goldthread and barberry, oregano, hu zhang, and turmeric. Toremifene (Acapodene) is a selective estrogen receptor modulator that eliminates high-grade PIN and reduces the incidence of prostate cancer. After 4 months of toremifene (60 mg/day orally for 4 months), 72% of men treated (versus 17.9% of controls) had no high-grade PIN on subsequent prostate biopsies.191 In another study, cumulative risk for prostate cancer was reduced in patients taking toremifene 20 mg in contrast with placebo (24.4% versus 31.2%) with an annualized rate of prevention of 6.8 cancers per 100 men treated.192 Among patients with no biopsy evidence of cancer at baseline and 6 months, the 12-month incidence of prostate cancer was reduced by 48.2% with toremifene 20 mg in contrast to placebo (9.1% versus 17.4%). The 20-mg dose was most effective, but the cumulative and 12-month incidences of prostate cancer were lower with each toremifene dose versus placebo (cumulative risk, 29.2% for 40 mg, 28.1% for 60 mg; 12-month incidence 14.3% for 40 mg, 13.0% for 60 mg).192 The final results at 3 years failed to yield a significant difference in cancer yield between untreated and treated patients (34.7% versus 32.3%, respectively).163 Green tea catechins also may reduce the incidence of prostate cancer in men with PIN. Catechins are antioxidants in the class of polyphenols called flavonols. After 6 months of green tea catechins 600 mg/day orally, 3.3% of the men with PIN had cancer in contrast to 30% of those who took placebo.193 The potent antioxidant lycopene at 4 mg/day was found in a prospective randomized placebo-controlled 1-year study of 40 men with PIN to significantly reduce the risk for subsequent prostate cancer, with no toxicity and good patient tolerance.194 In a prospective randomized 3-year trial of selenium versus placebo, Marshall and colleagues195
Neoplasms of the prostate
Epithelial neoplasms
Prostatic intraepithelial neoplasia
Epidemiology of prostatic intraepithelial neoplasia
Diagnosis of prostatic intraepithelial neoplasia
Useful immunohistochemical markers for the diagnosis of prostatic intraepithelial neoplasia

Differential diagnosis of prostatic intraepithelial neoplasia
Inflammation, atrophy, and high-grade prostatic intraepithelial neoplasia
Clinical significance of prostatic intraepithelial neoplasia
Prostatic intraepithelial neoplasia does not elevate prostate-specific antigen
Imaging cannot detect prostatic intraepithelial neoplasia
Prostatic intraepithelial neoplasia predicts coexistent or subsequent prostate cancer
Biopsy findings
Suggested response†
Isolated PIN (three or fewer sites)
Repeat biopsy after 1 year if PSA rises
Extensive or multifocal PIN (more than three foci)
Repeat biopsy
ASAP
Repeat biopsy
ASAP + PIN
Repeat biopsy
Intraductal carcinoma
Immediate repeat biopsy or definitive therapy‡
Clinical response and chemoprevention of high-grade prostatic intraepithelial neoplasia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree