Fig. 5.1
Operating room setup for thoracoscopic esophagectomy
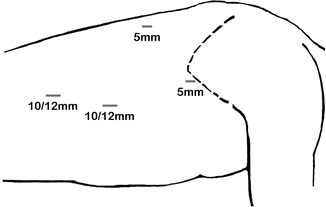
Fig. 5.2
Port placement for thoracoscopic esophagectomy
It is usually not necessary to divide the inferior pulmonary ligament and dissection of the esophagus starts from the mid-esophagus below the subcarinal area and then extends downward. The incision is made along the anterior portion of the esophagus. Using energy devices, such as electrocautery or Harmonic Scalpel (Ethicon Endo-Surgery, Cincinnati, OH), the anterior surface of the esophagus is dissected from the posterior portion of the pericardium and the anterolateral surface of the aorta. After completion of the anterior dissection, I prefer to place a small sponge between the aorta and the esophagus. This will facilitate subsequent posterior dissection. If the esophagus is pulled anteriorly, the sponge will be located between the esophagus and the aorta, and the surgeon can carry out dissection without the danger of injuring the thoracic aorta (Fig. 5.3). Once the circumferential dissection of the esophagus is accomplished, a penrose drain is placed around the esophagus and it is used to retract the esophagus by the assistant surgeon (Fig. 5.4). Small perforators from the thoracic aorta should be controlled using either endoclips or energy devices. All the para-esophageal lymph nodes are dissected en bloc with the esophagus. It is usually not difficult to expose the diaphragmatic crus because CO2 insufflation will push down the diaphragm and facilitate exposure.
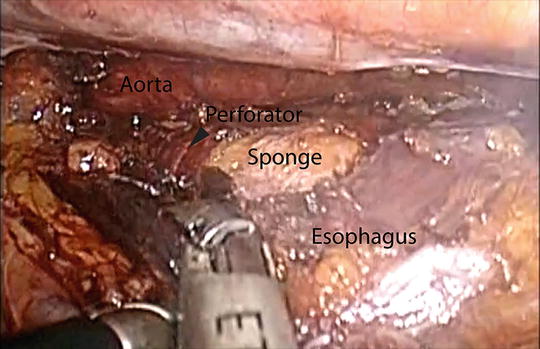
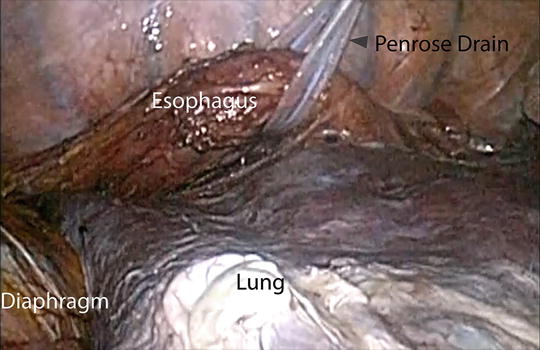

Fig. 5.3
Dissection of the lower thoracic esophagus with a sponge placed between the esophagus and aorta

Fig. 5.4
Retraction of the thoracic esophagus using a penrose drain
I prefer to resect the thoracic duct en bloc with the esophagus. In case the thoracic duct is not resected and if there is any suspicion of injury, I recommend clipping it at the most proximal portion. It is noteworthy that the thoracic duct runs separately from the azygos vein at the level of the lower esophagus and its course varies. However, the thoracic duct is easily found at the level of the mid-esophagus because it is running parallel with the azygos vein along its medial border. Once the thoracic duct is found, it is easy to dissect it proximally downwards and the most proximal end can be easily clipped.
Attention is directed to the mid-esophagus and the subcarinal lymph nodes are dissected en bloc with the esophagus (Fig. 5.5). Depending on the situation, one can dissect the subcarinal lymph nodes separately from the esophagus. The dissection is continued upwards to the azygos vein. Usually, there are many blood vessels at this level, supplying the esophagus as well as the peri-esophageal lymph nodes. These blood vessels should be carefully controlled. The mediastinal pleura above the azygos vein is then opened and the azygos vein is divided with an endostapler. Further dissection is carried out so that the esophagus is circumferentially mobilized from the esophageal hiatus up to the thoracic inlet. Above the level of the azygos vein, the dissection is maintained close to the esophagus to avoid potential injury to the left recurrent laryngeal nerve as well as the membranous portion of the trachea.
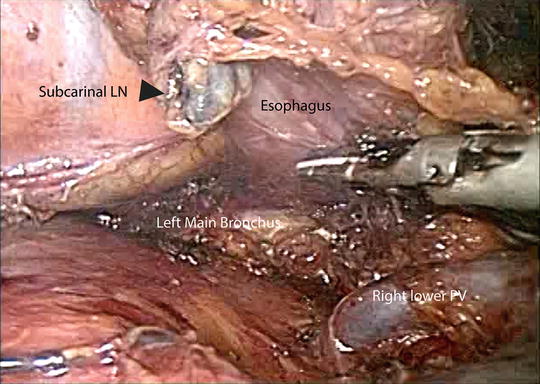

Fig. 5.5
Dissection of the middle thoracic esophagus and subcarinal lymph nodes
Mediastinal lymph node dissection is also performed. The left paratracheal lymph nodes are dissected with great care as not to injure the left recurrent laryngeal nerve. As it is difficult to dissect the left paratracheal lymph nodes, some surgeons suggest placing a single-lumen tube to facilitate exposure of the left paratracheal area. Personally, I prefer a double-lumen tube intubation. In case the left paratracheal lymph node dissection is difficult, I often make an upper partial sternotomy during the cervical procedure and dissect both paratracheal lymph nodes. It is especially beneficial because one can easily localize both recurrent laryngeal nerves and lymph node dissection can be carried out under the direct vision.
Some surgeons prefer to leave a penrose drain attached to the uppermost portion of the esophagus so that they can easily find the esophagus at the time of neck dissection. However, I find that it is not necessary. At the completion of the thoracoscopic procedure, a 28-French chest tube is inserted through the inferior port for postoperative chest drainage. The lungs are inflated and the port sites are closed.
Laparoscopic Gastric Conduit Preparation
The patient is placed in the supine lithotomy position. It is mandatory to take special care to avoid excessive abduction of the hip joint. The skin is prepped and draped from the neck to the pubic symphysis. The surgeon stands between the legs of the patient (Fig. 5.6). A 10-mm skin incision is placed in the midline above the umbilicus and abdominal insufflation is achieved by Veress needle. A 10-mm trocar is then placed through the same skin incision, and a 30° laparoscope is introduced. I prefer to use five abdominal trocars. Once the camera is placed, a thorough staging procedure is performed to determine if occult metastases are present. The second trocar (12 mm) is placed at the right anterior axillary line at the level of the umbilicus, for the liver retractor and stapler. A 5-mm trocar is placed at the right mid-clavicular line below the costal margin to be used by the surgeon’s left hand. A 5-mm trocar is placed at the left mid-clavicular line below the costal margin and used for the surgeon’s right hand. Another 5-mm trocar is placed at the left anterior axillary line at the level of the umbilicus for retraction by the assistant surgeon (Fig. 5.7). The patient is placed in reverse Trendelenburg position to help with exposure by displacing the stomach and colon caudally. The left lobe of the liver is retracted upward by placement of a liver retractor.
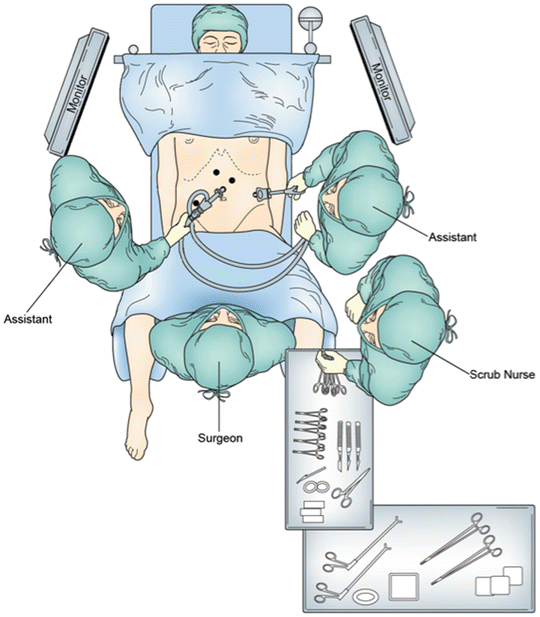
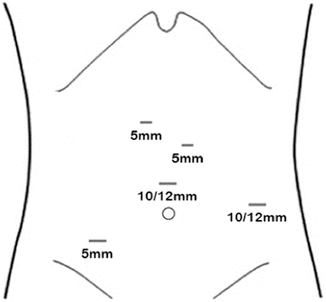

Fig. 5.6
Operating room setting for laparoscopic gastric conduit preparation

Fig. 5.7
Port placement for laparoscopic preparation of the gastric conduit
Using an endograsper in the left hand and an energy device in the right hand, the lesser sac is opened and divided up to the hiatus. To avoid losing pneumoperitoneum, I leave the dissection of the hiatus for last. The greater curvature of the stomach is prepared carefully to avoid injury to the right gastroepiploic vessels. The assistant surgeon pulls the greater omentum to the left. I prefer to start the dissection of the greater omentum at the 4 o’clock position because it is easy to find an area bare of omental vessels. I use an energy device for cauterization and division of the greater omentum. Further dissection is continued towards the gastrolienal ligament by dividing the short gastric vessels. During this procedure, the assistant surgeon may pull the stomach upwards and to the right to expose the space between the stomach and the spleen.
After completing preparation of the greater curvature, attention is directed to the left gastric vessels. The stomach is pulled upward and the surgeon approaches the left gastric vessels from the left in the space between the stomach and the pancreas. Alternatively, the stomach can be pulled to the left and the left gastric vessels can be approached from the right side of the lesser sac. The left gastric vessels are isolated and divided. One can either use vascular clips or a vascular endostapler for this procedure. The left gastric lymph nodes are resected en bloc with the surgical specimen. The posterior portion of the stomach is further dissected up to the hiatus. For the gastric drainage, some surgeons prefer pyloromyotomy or pyloroplasty. Some surgeons do not recommend a gastric drainage procedure [13]. However, I prefer injection of Botox (0.4 units) into the pylorus [14].
Cervical Esophageal Dissection
An oblique left neck incision is made and the platysma is divided. The sternocleidomastoid muscle is retracted laterally. The middle thyroidal vessels and the omohyoid muscle are divided. The thyroid gland is retracted medially and the posterior portion of the esophagus is dissected. It is important not to use metal retractors for the medial side retraction to avoid injury of the recurrent laryngeal nerve. A careful dissection is carried out between the upper trachea and the anterior border of the cervical esophagus to avoid injury of the recurrent laryngeal nerve as well as the superior laryngeal nerve. The cervical esophagus is encircled with a penrose drain, and blunt dissection is carried inferiorly to join the dissection plane achieved in the right chest (Fig. 5.8) [6].
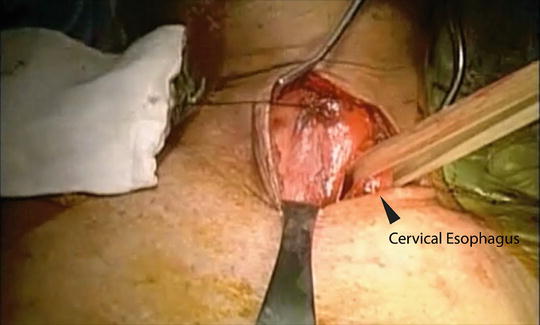

Fig. 5.8
Dissection of the cervical esophagus
Gastric Pull-up and Esophagogastric Anastomosis
After completion of the cervical esophageal mobilization, attention is directed to the abdominal cavity. The lesser curvature fat pad is removed at the fourth branch point of the right gastric artery. Starting from that area, multiple linear staplers are used to construct the gastric conduit (4–5 cm in diameter) (Fig. 5.9). The conduit is separated from the specimen at the angle of His. The esophagus is circumferentially mobilized at the esophageal hiatus. If needed, a portion of the right crus of the diaphragm is divided to enlarge the esophageal hiatus to facilitate exposure and transhiatal delivery of the surgical specimen. Under laparoscopic guidance, the surgical specimen is removed through the neck incision. A plastic bag is tied onto a Foley catheter. This Foley catheter is inserted to the neck wound through the posterior mediastinum and down to the hiatus. The distal portion of the gastric conduit is sutured onto the tip of the Foley catheter (Fig. 5.10). The gastric conduit is wrapped in the plastic bag and warm saline solution is poured into the bag. Wall suction is applied onto the Foley catheter and this results in vacuum wrapping of the gastric conduit. Using this method, the gastric conduit can gently be pulled up without trauma to the ascending gastroepiploic arcade and without spiraling of the conduit.
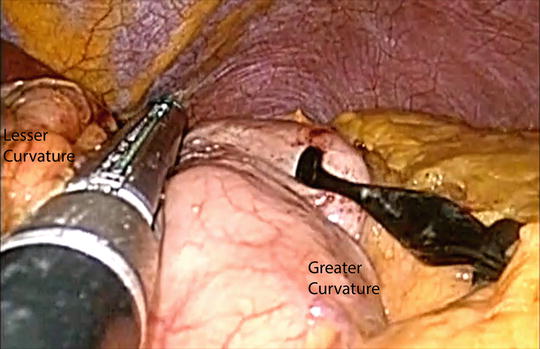
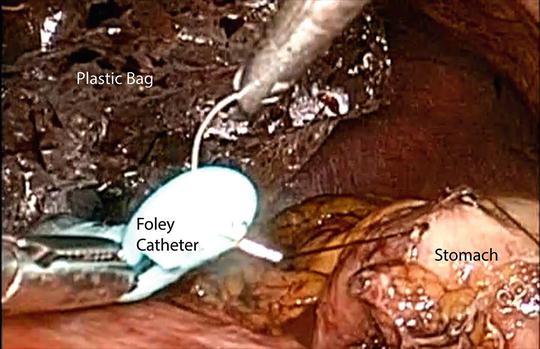

Fig. 5.9
Preparation of the gastric tube using serial firings of the endostapler

Fig. 5.10
Preparation for gastric pull-up. The gastric tube is sutured onto the tip of a Foley catheter
The cervical esophagus is divided at an appropriate level, and the resection margin is sent for frozen section examination. An esophagogastric anastomosis is performed with either hand-sewn technique or with endostaplers. A nasogastric tube is passed through the anastomosis. The neck is irrigated with antibiotic solution, and the wound is closed. The laparoscope is reinserted to inspect the abdominal cavity for adequate hemostasis. The ports are removed and the port sites are repaired as usual.
Postoperative Management
Many reports suggest that early extubation after esophagectomy is associated with reduced morbidity and decreased length of ICU stay [15, 16]. We prefer to extubate the patient either in the operating room or in the intensive care unit as soon as the patient is fully awake. It is often challenging to manage the fluid balance adequately after esophagectomy. Adequate circulating blood volume and systemic blood pressure is mandatory to maintain perfusion for the conduit. However, fluid overloading should also be avoided because it could lead to increased tissue edema and subsequent pulmonary edema, which may lead to pulmonary complications [17]. Urine output of 0.5 mL/kg/h during the first 24 h is acceptable [18]. Although pain is minimal after minimally invasive three-field esophagogastrectomy, intravenous patient-controlled analgesia is generally recommended in all patients.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








