Testicular cancer is the most common solid organ malignancy in young men between the ages of 15 and 35. Although much of this increase in survival can be attributed to improvements in systemic chemotherapy, surgery retains a critical role in the diagnostic and therapeutic management of testicular cancer. Laparoscopic retroperitoneal lymph node dissection is an effective staging and therapeutic procedure in patients with low-stage testicular cancer. It is an attractive alternative to the open approach, with faster recovery, improved cosmesis, and reduced post-operative morbidity driving its application. In experienced hands, it can be used in postchemotherapy patients.
This article is not certified for AMA PRA Category 1 Credit ™ because product brand names are included in the educational content. The Accreditation Council for Continuing Medical Education requires the use of generic names and or drug/product classes as the required nomenclature for therapeutic options in continuing medical education.
For more information, please go to www.accme.org and review the Standards of Commercial Support.
Histologically, all testicular cancer can be classified into germ cell and nongerm cell. Nongerm cell tumors do not exhibit typical, predictable retroperitoneal metastasis and are not the focus of this article. Germ cell tumors (GCT) are further divided into 2 groups, seminomatous and nonseminomatous.
The spread of GCTs is well described and highly predictable. Beginning within the parenchyma of the testis, the tumor cells can rapidly metastasize into the retroperitoneal lymph nodes. Laterality does predict which lymph nodes in the retroperitoneum are likely to be affected. This pattern of lymphatic spread allows for the creation of lateralized templates for localized therapy ( Figs. 1–3 ).
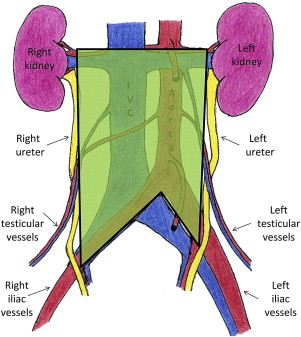
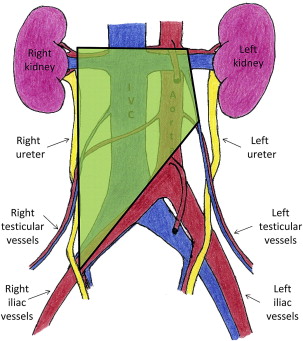
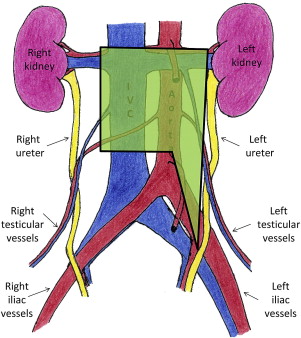
In seminomatous GCTs, radiotherapy plays a prominent role in treatment of retroperitoneal disease. These tumor cells are highly radiosensitive; therefore, they are often treated with radiation to the retroperitoneum for low-stage disease. Other options for low-stage (American Joint Committee on Cancer Stage 1, 2a, and 2b), seminomatous GCT include close observation protocols and single-agent chemotherapy. For higher-stage disease, multiagent chemotherapy is routinely used.
For nonseminomatous GCT’s (NSGCT), radiotherapy is not an option. After orchiectomy, therapeutic options are limited to close surveillance, chemotherapy, or retroperitoneal lymph node dissection. In choosing between these options, many factors, such as morbidity, cost, reliability, patient preference, and ability to tolerate surgery or nephrotoxic agents are increasingly important.
Retroperitoneal lymph node dissection (RPLND) provides essential staging information, which will in turn determine further therapeutic options. In certain patients, RPLND can also be therapeutic. For example, it eliminates the primary landing zone in clinical stage 1 patients and ensures complete removal of all residual retroperitoneal tissue after chemotherapy.
However, only 30% of patients who are clinical stage 1 for NSGCT show any positive nodes after RPLND. This could represent considerable overtreatment with a procedure that could cause considerable morbidities. Traditional open RPLND is associated with a large abdominal incision (xyphoid process to pubic symphysis) and several days of postoperative hospital stay. In addition, patients can potentially experience ejaculatory dysfunction due to dissection around the sympathetic nerves, although these rates have significantly decreased with the advent of nerve-sparing RPLND.
With the advent and widespread adoption of laparoscopic surgery, laparoscopic RPLND (L-RPLND) is increasingly being used as a treatment option for NSGCT. Aside from the obvious advantage of improved cosmesis from smaller incisions and shorter postoperative hospital stay, laparoscopy offers a highly magnified view of delicate retroperitoneal structures such as the sympathetic nerve plexuses. A recent meta-analysis suggests equivalent rates of staging accuracy and long-term disease-specific survival to open RPLND. Despite increased operating room time often required for this approach (204 vs 186 minutes in the recent meta-analysis), L-RPLND demonstrates benefits in length of stay, postoperative pain, and overall complication rates. With experience, therapeutic dissections equivalent to open surgery are achievable.
Indications
All patients with testicular cancer diagnosed after orchiectomy should have a complete metastatic evaluation. This includes comprehensive serum chemistries, liver function tests, bone scan, and computerized tomography of the chest, abdomen, and pelvis. Serum tumor markers specific to testicular cancer, namely α-fetoprotein, β-human chorionic gonadotropin, and lactate dehydrogenase, should be obtained after sufficient time for normalization following orchiectomy.
Current indications for RPLND are for patients with stage 1, stage 2a, and a subset of patients with low-volume stage 2b NSGCT. The procedure serves as a diagnostic procedure to stage the disease. In addition, given that most metastatic disease is confined to the retroperitoneum, excision of all retroperitoneal nodal tissue can be therapeutic. In a diagnostic capacity, RPNLD can identify patients with no evidence of lymphatic disease within the template of nodes removed. This can be very beneficial in patients who wish to avoid the toxicities associated with chemotherapy. However, in these patients, up to 10% will develop extraretroperitoneal recurrences, and they still require long-term imaging follow-up, especially in the chest and mediastinum.
RPLND not only has a role in primary treatment after diagnostic orchiectomy, but also as a salvage treatment option after chemotherapy. A common problem for the clinician is the residual retroperitoneal mass after chemotherapy for testicular cancer. Upon excision of residual masses after chemotherapy, approximately 40% will contain teratoma, and 20% will contain viable tumor, both requiring excision for cure. The remaining 40% will have necrotic tissue. Unfortunately, current imaging techniques are unable to reliably differentiate necrotic tissue from teratoma or viable tumor.
Patients not suitable for RPLND include those who have clinical stage 2 NSGCT with elevated tumor markers, because this usually reflects systemic disease. Also indicative of systemic disease is the presence of enlarged lymph nodes in the suprahilar, retrocrural, pelvic, inguinal, or contralateral areas. In addition, when considering L-RPLND, patients with a bleeding diathesis, bulky lymphadenopathy, or active peritoneal or abdominal wall infection should be avoided. Also, in postchemotherapy patients undergoing L-RPLND, the dense scar tissue can make laparoscopic dissection challenging. Subsequently, higher rates of conversion to open RPLND are seen in this population.
Preparation
Patients should be extensively counseled about the various treatment options available, including initial chemotherapy, surgery, and when appropriate, surveillance. A thorough discussion of known complications should include damage to the gastrointestinal tract, kidneys, liver, and pancreas. In addition, manipulation of the sympathetic chain may cause postoperative neurologic complications and ejaculatory disturbance. Consequently, patients should be encouraged to pursue preoperative sperm banking if interested in having biologic children.
Some have advocated starting patients on a low-fat diet 2 weeks before surgery to reduce the chance of chylous ascites. The day before surgery, a mechanical bowel prep is very helpful, and if anticipating multiple adhesions, a full bowel preparation should be considered. Type and screen are essential for all patients.
Postchemotherapy patients present special challenges due to the widespread use of bleomycin, which can put patients at risk for pulmonary fibrosis. This bleomycin-induced pulmonary fibrosis can cause multiple postoperative difficulties with respiration and ventilation, most notably for acute respiratory distress syndrome (ARDS). Thorough screening with pulmonary function testing and possible consultation with a pulmonologist preoperatively should be considered. In these patients, intraoperative intravenous fluid administration should be closely monitored due to the increased postoperative pulmonary difficulties.
It is also important to remember the effects of myelosuppression when planning the timing of surgery after chemotherapy. A period of at least 5 weeks should pass for proper cellular regeneration before surgery. In addition, the use of nephrotoxic agents should be limited in patients who have undergone cisplatin-based chemotherapeutic regimens.
Preparation
Patients should be extensively counseled about the various treatment options available, including initial chemotherapy, surgery, and when appropriate, surveillance. A thorough discussion of known complications should include damage to the gastrointestinal tract, kidneys, liver, and pancreas. In addition, manipulation of the sympathetic chain may cause postoperative neurologic complications and ejaculatory disturbance. Consequently, patients should be encouraged to pursue preoperative sperm banking if interested in having biologic children.
Some have advocated starting patients on a low-fat diet 2 weeks before surgery to reduce the chance of chylous ascites. The day before surgery, a mechanical bowel prep is very helpful, and if anticipating multiple adhesions, a full bowel preparation should be considered. Type and screen are essential for all patients.
Postchemotherapy patients present special challenges due to the widespread use of bleomycin, which can put patients at risk for pulmonary fibrosis. This bleomycin-induced pulmonary fibrosis can cause multiple postoperative difficulties with respiration and ventilation, most notably for acute respiratory distress syndrome (ARDS). Thorough screening with pulmonary function testing and possible consultation with a pulmonologist preoperatively should be considered. In these patients, intraoperative intravenous fluid administration should be closely monitored due to the increased postoperative pulmonary difficulties.
It is also important to remember the effects of myelosuppression when planning the timing of surgery after chemotherapy. A period of at least 5 weeks should pass for proper cellular regeneration before surgery. In addition, the use of nephrotoxic agents should be limited in patients who have undergone cisplatin-based chemotherapeutic regimens.
Operating room setup
The surgeon stands on the contralateral side of dissection and is encouraged to use a self-retaining laparoscopic camera-holding device (robotic or mechanical) to prevent crowding from the assistant. Monitors are placed on each side, so both surgeon and assistant have unobstructed, ergonomic viewing angles of the images. A full laparoscopic tray, including vascular instruments, should be available. In addition, a full open vascular tray should be in the operating room and immediately available for timely conversion to the open procedure if necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







