PBC: primary biliary cirrhosis; OLT: orthotopic liver transplantation; CAH: chronic active hepatitis; ALD: alcoholic liver disease; HA: hydroxyapatite; NaF: sodium fluoride; UDCA: ursodeoxycholic acid; DPA: dual photon absorptiometry; DXA: dual X-ray absorptiometry; PBA: photon beam absorptiometry; SPA: single photon absorptiometry; LS: lumbar spine; FN: femoral neck; 25-OH-D3: 25-hydroxy-vitamin D3; PO: per os; SC: subcutaneous; IM: intramuscular.
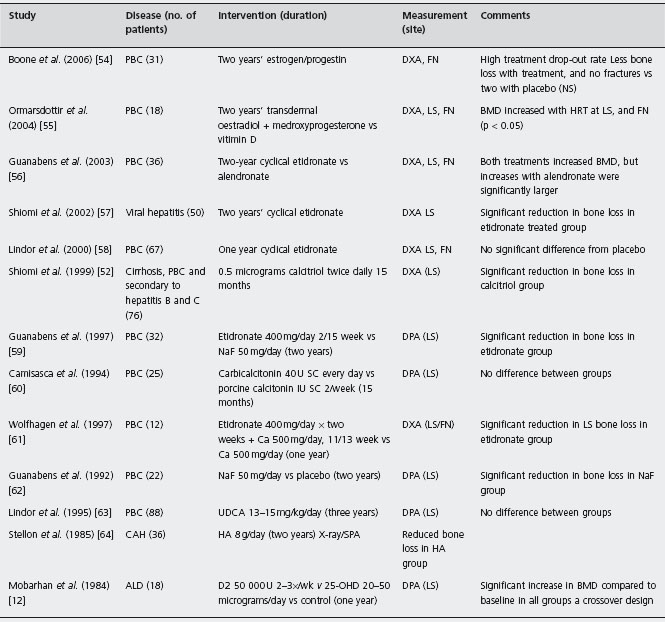
Wolfhagen et al. compared etidronate plus calcium with calcium alone in a randomized trial in 12 women with PBC on corticosteroids [61]. There was a statistically significant difference in the percentage change in mean lumbar BMD between the etidronate and calcium-treated groups (etidronate + 0.4%, calcium –3.0%, p = 0.01) [61]. Ald In a randomized trial that was not blinded, Shiomi et al. evaluated etidronate in 45 women with cirrhosis due to underlying viral hepatitis and also found a statistically significant difference in the percentage change in lumbar spine BMD [57]. Guanabens et al. compared alendronate with etidronate in an RCT of 36 women with PBC [56]. After two years, both treatments increased bone density but the increase was significantly greater in women on alendronate. Ald
Camisasca et al. [60] evaluated the effect of a six-month course of calcitonin 40IU every other day, given subcutane-ously in a trial with a crossover design. The control group received 1 IU of porcine calcitonin (no metabolic effect). Both groups received calcium and 100,000 IU of parenteral vitamin D2 (n = 25). Treatments were administered for six months with a three-month washout. There was no difference in bone density between the two treatment groups in either of the crossover periods. It is possible that this study was inadequately powered to detect a statistically significant difference. Ald
In another trial of 22 women with PBC followed for two years, Guanabens et al. [62] compared NaF to calcium in a two-year RCT. In the NaF group, the bone density of the lumbar spine increased by 2.9% compared with the control group in which it decreased by 6.6%. However, there was a high frequency of adverse effects, mainly gastrointestinal. Since NaF therapy was also less effective than etidronate in another study, this intervention is not recommended.
In summary, both cholestatic and non-cholestatic types of liver disease may be complicated by metabolic bone disease, predominately osteoporosis. The prevalence of bone disease increases with the degree of cirrhosis. Accelerated bone loss is most severe after liver transplantation. Patients with advanced liver disease, awaiting transplantation, on prolonged corticosteroid therapy or with a history of fragility fractures, should be investigated with BMD testing. C5 Low 25-(OH) D3 levels should be corrected and calcium supplementation given. Bisphosphonates should be considered in patients with known osteoporosis or vertebral fractures, based on the evidence from a number of small randomized trials. Ala Testosterone therapy should be considered in males with hypogonadism. C5
PBC: primary biliary cirrhosis; OLT: orthotopic liver transplantation; CAH: chronic active hepatitis; ALD: alcoholic liver disease; HA: hydroxyapatite; NaF: sodium fluoride; UDCA: ursodeoxycholic acid; DPA: dual photon absorptiometry; DXA: dual X-ray absorptiometry; PBA: photon beam absorptiometry; SPA: single photon absorptiometry; LS: lumbar spine; FN: femoral neck; 25-OH-D3: 25-hydroxy-vitamin D3; PO: per os; SC: subcutaneous; IM: intramuscular.
There is a need for population-based studies of fracture risk in patients with chronic liver disease.
Inflammatory bowel disease
Prevalence
The importance of metabolic bone disease in patients with IBD has been recognized for some time. However, the point prevalence of bone disease in this population varies greatly from one study to another, with estimates as low as 5% to as high as 78%. This variation reflects a number of factors, including the definition of osteoporosis used, the site of bone density measurement and the heterogeneous nature of the IBD population. Furthermore, morphometric vertebral deformities also appear to be relatively common and are seen in 25% of both Crohn’s disease, and ulcerative colitis patients [65]. A list of potential factors that need to be considered when evaluating studies in this area is provided in Table 16.3.
In a well-conducted study, Abitbol et al. [66] evaluated the BMD of 84 consecutive patients with IBD (34 Crohn’s disease, 50 ulcerative colitis, excluding proctitis). Overall, 43% had osteopenia in the lumbar spine. Steroid users were at significantly greater risk of osteopenia (58% vs 28% in non-users, p = 0.03). Six patients with a mean age of 50 had vertebral crush fractures (mean Z-score was –1.63). Five patients were found to have low 25(OH) D3 levels; however, the cause of this deficiency was felt to be extra-intestinal in all but one case. Multiple regression analysis of the lumbar spine Z-score revealed a significant correlation between osteopenia and age, cumulative corticosteroid dose, inflammatory status as assessed by the erythrocyte sedimentation rate (ESR), and low osteocalcin levels (r2 = 0.76, p < 0.05).
The rate of bone loss in IBD has been studied in several longitudinal studies [67–73]. In the majority of the studies the annual rate of bone loss appears to be greater in the spine than at the radius, and varies from 2% to 6%. Schulte et al. studied the rate of BMD change in 80 IBD patients and found that the annual rate of bone loss was small (0.8%/ year for spine) [71]. Corticosteroid use [67] and low body mass index [68] were found, in some studies, to affect bone mass negatively. Overall, metabolic bone disease is an important problem among patients with IBD, with an estimated prevalence in the range of 45%.
Table 16.3 Factors influencing interpretation of studies of bone disease in gastrointestinal patients.
| Definition of osteopenia | Z-scores of ≤ 1 (? T-scores of –1 to–2.5) |
| Diagnostic method | X-ray, SPA, DPA, QCT, DXA, US |
| Results expressed (outcome) | BMD, BMC, radiological or clinical fracture (vertebral or non-vertebral) |
| Bone site studied | Spine, forearm, femoral neck or total hip |
| High risk patients | Included or excluded |
| Control of confounders | Smoking, steroid use, BMI |
SPA: single photon absorptiometry; QCT: quantitative computed tomography; DPA: dual photon absorptiometry; DXA: dual energy X- ray absorptiometry; BMD: bone mineral density; BMC: bone mineral content.
Malabsorption of calcium and vitamin D because of small bowel disease appears to play a minor role in the pathogenesis of metabolic bone disease in IBD. Both low and normal 25(OH) D levels have been documented in patients with Crohn’s disease and there is no clear correlation between vitamin D levels and bone mass [74, 75]. Osteomalacia appears to be much less common than osteoporosis in IBD. Hessov et al. did a bone biopsy and serum 25(OH) D determinations on 36 randomly selected Crohn’s disease patients with previous surgical resections (mean length 105 cm). Only two patients were found to have below normal 25-hydroxy-vitamin D3 levels and/or histo-morphometric evidence of osteomalacia. However, the mean trabecular bone volume was reduced in this group compared with controls, suggestive of osteoporosis. This finding did not correlate with any of the measured clinical characteristics, including length of resection and serum 25(OH) D level [76]. Another bone histomorphometry study in IBD revealed decreased bone formation without evidence of osteomalacia [77].
Comparisons between Crohn’s disease and ulcerative colitis patients suggest that osteoporosis may be more prevalent in the former [78–80]. However, careful review of these publications suggests that the analysis may not have been fully controlled for the effects of disease activity and/or steroid use. Jahnsen et al. in an age and sex-matched cross-sectional study of 60 Crohn’s disease patients, 60 ulcerative colitis patients and 60 controls, found no differences in BMD between the patients with ulcerative colitis and the controls [79]. However, Crohn’s disease patients had significantly lower BMD. Overall 16% of ulcerative colitis patients and controls had Z-scores <–l compared with 23% of Crohn’s disease patients [79]. However, significantly more Crohn’s disease than ulcerative colitis patients used corticosteroids (72% vs 47%), and smoked (57% vs 28%). Although disease activity was not specifically addressed in this study, 53% of the ulcerative colitis group had left-sided disease, with 40% having proctosig-moiditis or less. As well, the BMD of Crohn’s disease patients who were not using steroids was not significantly different from that of the other two groups. Ghosh et al. [78] evaluated 30 IBD patients at the time of diagnosis and found that those with Crohn’s disease had significantly lower bone density than those with ulcerative colitis. The mean lumbar spine Z-score for Crohn’s disease patients was –1.06 versus –0.03 for those with ulcerative colitis. However, 7 of 15 ulcerative colitis patients had proctitis alone, and one had a “distal colitis”. As well, the mean duration of disease before diagnosis (18.6 vs 12 weeks), and of steroid use (1.2 vs 0.5 weeks) before BMD, measurements are slightly longer in the Crohn’s group, again suggesting that disease severity rather than diagnosis may be the important factor. Bernstein et al. in a study of 26 Crohn’s disease and 23 ulcerative colitis patients, also found a greater prevalence of osteopenia among the former [80]. However, using stepwise discriminant analysis, the authors found that steroid use rather than disease type was the most important predictive factor of osteopenia.
A cross-sectional study of 51 Crohn’s disease, 40 ulcerative colitis patients and 30 age and sex-matched controls by Ardizzone et al. found no significant difference in mean T-score values between patients with Crohn’s or ulcerative colitis but did find that 37% of Crohn’s and 18% of ulcerative colitis patients were osteoporotic based on WHO criteria [81]. Stepwise regression showed that in Crohn’s disease, the femoral neck T-score was inversely related to disease duration and lumbar spine T-score was inversely related to age. Reffitt et al. also found that BMD improved with increasing duration of remission and found a benefit with azathioprine induced remission [82]. There were baseline differences in disease duration between the two groups. Schulte studied the rate of BMD change in 80 patients with IBD. The results indicated that the average annual rate of bone loss was small. There was a large range in BMD results in these patients, suggesting that certain subgroups may lose bone more quickly than others.
The study of metabolic bone disease in ulcerative colitis before and after restorative proctocolectomy also suggests that disease activity plays an important role, since BMD increases significantly with time after colectomy, with a mean annual increase of around 2% [83].
Fracture prevalence estimates in IBD from cross-sectional and prospective studies have been variable, with larger series reporting vertebral fractures in 7–22% [84] and non-vertebral fractures in 27% of patients, although these data may have been affected by referral bias. There have been three population-based studies of fracture risk in IBD. Bernstein et al. identified 6027 IBD patients through an administrative database in a Canadian population and matched them to 60,270 controls by age, sex and geographic residence [85]. The overall fracture rate was higher compared with controls with a 41% overall increased incidence of hip, spine, wrist and rib fractures among IBD patients (RR 1.41, 95% CI: 1.27–1.56). The incidence rate ratio was 1.59 (95% CI: 1.27–2.00) for hip fractures. There were no difference in fracture rates between males and females or between Crohn’s and ulcerative colitis patients, except that males with ulcerative colitis had a higher fracture rate than females. Although the fracture risk of IBD patients was higher than controls, the increase was one patient per 100 patient years. Another North American study in Olmsted County, Minnesota assessed fracture risk in 238 Crohn’s disease patients through a review of radiology reports and found that compared with age and sex-matched controls the overall risk ratio for any fracture was 0.9 (95% CI: 0.6–1.4), which was not statistically significant. The risk ratio for vertebral fracture was 2.2 (95% CI: 0.9–5.5), and the relative risk for an osteoporotic fracture was 1.4 (95% CI: 0.7–2.7), all statistically non-significant [86]. Age was the only significant predictor of fracture risk in a multivari-ate analysis and fracture risk was not increased in comparison with the general population except in the elderly patients. The findings were similar for the ulcerative colitis patients. Vestergaard and Mosekilde in a population-based study from hospital discharge data did not find an increase in fracture risk except for a small increase risk of fracture that required hospitalization in Crohn’s disease patients [87]. The difference from fracture rates seen in ulcerative colitis patients was not statistically significant. A potential weakness of this study was the use of administrative databases which could result in the underreporting of fractures that do not require hospitalization. In addition, the diagnosis of Crohn’s disease and ulcerative colitis was only validated in a small sample of patients.
A recent nested case-control study of 231,778 fracture cases from the UK General Practice Research Database demonstrated an increased risk of vertebral (OR 1.72; 95% CI: 1.13–2.61) and hip fracture (OR 1–59; 95% CI: 1.14–2.23) in patients with IBD. There was a greater risk of hip fracture seen in Crohn’s disease patients compared with ulcerative colitis patients. This study also noted that only 13% of IBD patients who had already sustained a fracture were on osteoporosis treatment [88]. Corticosteroid use was associated with an increased risk of fracture and this association persisted after adjustment for disease severity (OR 1.10; 95% CI: 1.00–1.20). Limitations of this study included the method used to ascertain fractures and the fact that only clinically diagnosed fractures were included.
The literature suggests that there is a discrepancy between BMD findings and fracture risk in the IBD population and that the greatest risk is in elderly patients with IBD.
Treatment
Clements et al. in an uncontrolled two-year prospective study of HRT in 47 postmenopausal women with IBD (25 ulcerative colitis, 22 Crohn’s disease), found that radial and spine BMD rose significantly over baseline with HRT [89]. B4 The authors found no differences in the responses between patients with ulcerative colitis and Crohn’s disease. Patients using corticosteroids also seemed to respond.
Vogelsang et al. randomized 75 Crohn’s disease patients, without short bowel syndrome to either 1000IU vitamin D3 + calcium or placebo. The BMD of the forearm decreased in 80% of the control group compared to 50% of the treatment group at one year. BMD decreased less in calcium/ vitamin D-treated patients (median decrease in BMD: treated 0.2%, interquartile range 3.8–(+14)%; control 7%, range 12.6–(+14)%; p < 0.005). Ald The correlation between the change in vitamin D and change in BMC was low (r = 0.19) [90]. Bernstein et al. [91] in a pilot study of 17 IBD patients with a history of steroid use (14 men, 10 Crohn’s disease), assessed the efficacy of calcium supplementation (1000 mg/day) and vitamin D 250 IU on BMD by DXA. The authors found that the dose of prednisone in the year prior to the study inversely correlated with bone density at the hip, but not at the lumbar spine. There was no effect on bone density demonstrated after one year. Ald However, there is a significant risk of a type 2 error in this small study.
Robinson et al. [92] assessed the effect of low impact exercise in a randomized controlled trial. Although no statistically significant increase in BMD was observed in the exercise group, secondary analysis revealed that the number of exercise sessions correlated significantly with increased BMD at the hip and spine. Ald
Haderslev et al. assessed the impact of alendronate in Crohn’s disease patients with osteopenia in a 12-month RCT. Alendronate increased the BMD of the lumbar spine by 5.5% compared with control over a one-year period. Fractures were not evaluated [93]. Bartram et al. similarly evaluated IV pamidronate + calcium/vitamin D compared to calcium/vitamin D alone in an RCT and found that while both groups showed improvement in BMD of the LS and hip, the improvement with pamidronate was statistically significantly higher (2.6% vs 1.6% at the LS) [94]. Another bisphoshonate, risedronate also appears to be effective at improving BMD at the hip and LS [95]. However, in another study of 154 Crohn’s disease patients, Siffledeen et al. found that the addition of etidronate to calcium/ vitamin D did not further improve BMD over that seen with calcium and vitamin D alone [96]. Ald
von Tirpitz et al. studied the effectiveness of NaF (75 mg SR) on 33 subjects with Crohn’s disease in a 12-month RCT. The results indicated that NaF is effective at increasing mean spine Z-score (p = 0.02). In contrast, the control arm of calcium 1000mg/day and vitamin D 1000IU/day did not result in increases in spine BMD [97]. Ald
Biological therapies are now becoming commonplace for the treatment and maintenance of patients with IBD. The use of anti-TNF therapies appears to improve bone mineral density in IBD patients [98–101]. This effect may stem from several properties of these agents, such as: improvement in disease activity per se, reduction in inflammatory mediators, reduction in corticosteroid use, or a direct bone antire-sorptive effect. To study the relative effect of these factors, Pazianas et al. conducted a retrospective cohort study and found a greater improvement in BMD in IBD patients taking infliximab with a bisphosphonate compared to a bisphosphonate alone. This effect was inhibited by corticosteroid use. The authors did not find an independent effect of infliximab alone, suggesting that a primary effect on bone is likely not the main mode of action of this agent [102].
In summary, osteopenia is an important problem among patients with IBD, even at initial diagnosis. The risk appears to be greatest among those with the greatest disease activity and duration, and those treated with corticosteroids. It is difficult to distinguish the impact on bone density of corticosteroid use from that of disease activity, since these factors are linked. The risk of osteoporosis and facture in ulcerative colitis is similar to that seen in Crohn’s disease and after proctocolectomy the bone density of ulcerative colitis patients increases. The risk for osteoporosis and fractures appears to be similar in males and females. Crohn’s and ulcerative colitis patients seem to have comparable risks for fracture; although the overall rate for fracture is increased, the rate is affected by age.
There is evidence that IBD patients with low BMD benefit from a combination of vitamin D and calcium. HRT may be a less attractive option based on results from the Women’s Health Initiative study and concerns about an unfavorable risk profile, including an increased risk of breast cancer. Existing studies have used surrogate outcome measures, such as BMD, and management has been frequently been based on results from postmenopausal osteoporosis treatment trials. Further studies are needed to assess the impact of bisphosphonates on the clinically important outcome of fracture in the IBD population. Current recommendations are that for individuals with T-scores < –2.5 or vertebral compression fractures, therapy should include calcium and vitamin D, in addition to bisphosphonates. Ald For those with T-scores between –2.5 and –1.0, therapy should include calcium, vitamin D and bisphosphonate therapy for patients on prolonged corticosteroid therapy. In patients with active Crohn’s disease parenteral administration of a bisphosphonate such as pamidronate or zoledronic acid may be indicated. C
Celiac disease
Prevalence
Osteoporosis
Prevalence rates of osteoporosis in celiac disease vary depending on the population studied (adults vs children) and whether the disease has been treated. Lower BMD values have been noted in untreated populations, including those individuals who are asymptomatic at presentation. A number of cross-sectional studies have evaluated the prevalence of osteoporosis in (1) newly diagnosed celiac patients and (2) individuals treated with a gluten-free diet [103–110]. In general, studies in untreated celiac disease demonstrate diminished bone density. When compared with age-matched controls (Z-score of <–2 at the spine), the prevalence of osteopenia in untreated patients varied among studies from 15–40% [105,108]. Serum PTH levels have been shown to correlate inversely with BMD [105,109] and levels of 25(OH) D correlate positively with BMD in untreated celiac disease patients [109].
Fractures
Vasquez et al. estimated the incidence of fractures from a case-control study (ascertained fractures by interview) and found that 25% of patients had a history of previous fractures, compared with 8% of age and sex-matched controls (odds ratio (OR) 3.5; 95% CI: 1.8–7.2), with the majority of fractures occurring prior to diagnosis or in those individuals who were non-compliant [110]. The most common fracture was a wrist fracture and there was a trend to increased vertebral fractures. Vasquez studied patients from a mal-absorption clinic and therefore this rate may not be representative of the general celiac population. Neither BMD or body mass index correlated with the presence of fractures, suggesting that there are other factors beside BMD, such as disease duration, that account for increased fracture risk.
Vestergaard and Mosekilde (2002) in Denmark in a retrospective cohort study examined hospital discharge abstracts for patients previously hospitalized with a diagnosis of celiac disease and did not detect a significant difference in fracture rates compared with controls [87]. Age was the only significant risk factor for fracture. There are potential sources of bias in using hospital-based discharge data including the fact that outpatient fracture diagnoses are not included. Thomason et al. (2003) in a case-control study in which fractures were ascertained using a questionnaire did not find an increased fracture risk in celiac disease patients compared with controls [111], and other small longitudinal studies have yielded similar findings [106, 112].
A recent systematic review and meta-analysis identified 60 potential studies of which eight met the inclusion criteria for the meta-analysis (e.g. outcome of fractures and controlled studies). The pooled prevalence of fractures in celiac disease patients was 8.7% (1819/20,955) compared to 6.1% (5955/96,777) for controls for a pooled odds ratio = 1.43; 95% CI: 1.15–1.78) although significant heterogeneity was observed (I2 = 85.1%), as evidenced by poor overlapping of confidence intervals for individual studies [113]. Six of the eight studies showed a significant association between fractures and celiac disease.
The largest included study in the meta-analysis was a retrospective population based cohort study by Ludvigsson et al. (2007) that used the Swedish National Registers to estimate the risk of hip and any fracture. They found that individuals with celiac disease were at increased risk of hip fracture (HR 2.1; 95% CI: 1.8–2.4) and for any type of fracture (HR 1.4; 95% CI: 1.3–1.5) [114]. Limitations include the inability to control for all potential confounding variables such as body mass index, smoking and the use of bisphos-phonates or hormone replacement therapy. West et al. (2003) in another large retrospective cohort study in the UK also found a significant increase in fractures in celiac disease patients [115].
Further clarification of the risk of fracture in celiac disease with a large prospective cohort study with rigorous ascertainment of fractures would be helpful.
Pathogenesis
Reduced calcium absorption can result in hypersecretion of PTH, enhanced 1,25-dihydroxy vitamin D and decreased 25 hydroxy-vitamin D [116]. In addition, systemic inflammatory effects may result in bone loss via action of inter-leukin (IL)-1 and IL-6, the levels of which have been shown to correlate with BMD [117]. It is also thought that zinc deficiency may lead to reduced IGF-1 levels which in turn results in impaired bone metabolism and reduced bone mass [118]. It is not clear what proportion of individuals with celiac disease have osteomalacia, due to the lack of bone biopsy data, but many individuals are vitamin D deficient.
Treatment
Longitudinal studies of patients with celiac disease have demonstrated increases in BMD after starting on a gluten-free diet and the majority of the change occurs within the first year, particularly at the lumbar spine [119]. B4 The average increase in lumbar spine BMD is approximately 5% within the initial year. A number of observational studies have shown that children will often normalize their BMD after a gluten-free diet [119–122]. B4 Adults, however, may continue to have BMDs below average (Z-score of –1 at the spine) [106,123]. Premenopausal females have shown a greater increase in BMD than postmenopausal females.
Valdimarsson et al. found that patients with secondary hyperparathyroidism at baseline did not increase their BMD to normal by three years in comparison to those who had a normal baseline PTH, and did achieve a normal BMD [105].
The goal of treatment should be to maintain normal serum 25(OH) D levels, with vitamin D supplements and higher doses may be required initially. Bone density scans should be recommended in newly diagnosed adult celiac patients after one year on a gluten-free diet. Initial evaluation should also include serum calcium, 25(OH)D and PTH levels. Additional osteoporosis therapies may be considered depending on the severity of bone loss.
Glucocorticoid-induced bone loss
Glucocorticoids are widely used in the treatment of inflammatory bowel disease, and are an important independent risk factor for bone loss. Observational studies have demonstrated an association between cumulative corticosteroid dose and increased bone loss, in multiple populations, but some prospective studies have failed to support this relationship, perhaps because of a beneficial effect of corticos-teroids on disease activity [124].
Data from cross-sectional studies of patients on corticos-teroids estimate that the incidence of fractures varies from 30% to 50% [84, 125]. In a study by Adinoff and Hollister, 11% of asthma patients on oral steroids for one year developed vertebral fractures [126]. In a case-control study, Cooper et al. (Van Staa et al. [127]) found that use of oral steroids resulted in an RR of 1.16 (CI: 1.47–1.76) for hip fracture and 2.6 (CI: 2.31–2.92) for vertebral fracture. A nested case-control study from the Study for Osteoporotic Fractures Cohort confirmed an increase incidence of hip fractures in patients on corticosteroids with an adjusted relative risk of hip fracture of 2.1 (95% CI: 1.0– 4.4) [128]. A meta-analysis of seven prospective cohort studies, found that previous GC use was associated with an RR of 2.63 of sustaining an osteoporotic fracture and 4.42 for hip fracture after adjusting for BMD [129]. The risk of fracture is increased at higher doses of corticosteroids.
There is evidence that the relationship between bone density may underestimate the risk of fracture in patients on corticosteroids, highlighting the importance of bone quality [130, 131]. Glucocorticoid-induced bone loss is greatest in the initial 6–12 months of treatment [132–134], and involves areas of the skeleton which have the greatest turnover, in particular, the lumbar spine, cortical rim of the vertebral body, ribs and proximal femur. Hahn et al. demonstrated that trabecular bone loss is greater than cortical bone loss in rheumatoid arthritis patients on prednisone [135]. Fracture risk increases within the first 3–6 months of glucocorticoid treatment [131].
Pathogenesis
The mechanism of corticosteroid-induced osteoporosis (CSOP) is multifactorial [139]. CSOP differs from postmen-opausal osteoporosis in that bone formation is greatly decreased at a time of increased bone resorption. This imbalance between formation and resorption results in a reduction in the total amount of bone replaced in each remodeling cycle. Corticosteroids cause a reduction in bone formation by decreasing the production of osteoblasts and increasing the apoptosis of osteoblasts and osteocytes [140]. Corticosteroids also prolong the lifespan of mature osteoclasts, resulting in bone resorption [133]. Glucocorticoids also suppress the hypothalamic-pituitary-gonadal axis that leads to a functional hypogonadism and increased bone loss [141]. Finally, steroids cause loss of muscle mass, decrease the intestinal absorption of calcium and increase renal excretion of calcium.
One explanation for the rapid bone loss seen with corticosteroids could be related to the increase bone resorption that occurs secondary to the down-regulation of osteopro-tegerin (OPG), an osteoblast-derived soluble decoy receptor. OPG blocks the interaction between RANK and RANK-L (receptor activator of nuclear factor ΚB ligand) that inhibits osteoclast formation and also increases expression of RANK-L by osteoblasts [142]. The result is a decrease in the OPG/RANK-L ratio leading to an increase in cancel-lous osteoclasts and rapid bone loss [143].
Prevention and treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree