Table 43.1 Circumstances affecting the net state of immunosuppression in liver transplant recipients (adapted from reference 11).
| Pharmacological immunosuppression | ||
| Invasive manoeuvres compromising muco-cutaneous barrier integrity: | ||
| Intravenous lines | ||
| Tracheal intubation | ||
| Reoperation | ||
| Bladder catheterization | ||
| Underlying immunomodulatory conditions (pre- and/or post-operative): | ||
| Very advanced liver disease | ||
| Metabolic: | ||
| Renal failure | ||
| Diabetes mellitus | ||
| Malnutrition | ||
| Multiple organ failure | ||
| Viral infection: | ||
| Cytomegalovirus | ||
| Epstein-Barr virus | ||
| Hepatitis C virus |
Beyond month six post-transplantation, and coinciden-tally with a progressive reduction of immunosuppression and little requirement of re-hospitalization, the risk of infection drastically decreases, becoming similar to that in the general population. During this late period, most infections are community acquired, although opportunistic infections can also occur.
This spectrum of infection occurrence can be altered due to a variety of reasons, with important overlap between the periods. For example, biliary and graft vascular problems can persist beyond the early postoperative period, with the subsequently prolonged risk of infection related to these surgical complications. The need to maintain long-term immunosuppression due to late-onset or persisting rejection is associated with an increased risk of opportunistic infections and latent infection reactivation throughout the time under potent pharmacological immunosuppression. Finally, the persistence or de novo appearance of immunomodulatory events, such as chronic renal failure or severe recurrence of pre-transplant liver disease (particularly, hepatitis C), can modify the risk of infection during the intermediate and late post-transplant periods.
Bacterial infection
Incidence and special risk factors
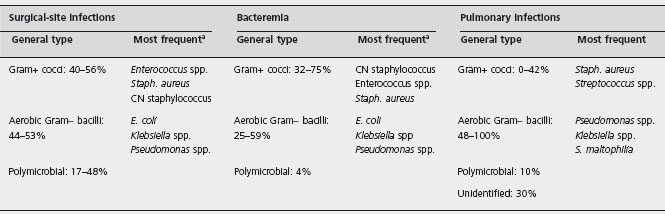
Bacterial infections are the most frequent infectious complications in liver transplant recipients and predominantly occur during the early postoperative period. The commonest bacterial infections in this period are surgical site infection, bacteremia and pneumonia. In most studies, the development of bacteremia and pneumonia has been associated with increased risk of mortality [17–22]. Surgical site infection, although not greatly influencing survival, increases hospital resource utilization and cost [23, 24]. In recent reports, the mean incidence of surgical site infection, involving the incision wound and intra-abdominal organ-space infections (namely, localized or diffuse peritonitis, cholangitis, infected biloma and hepatic abscess), is approximately 25%, ranging from 5% to 40% [23–33]. Bacteremia has also been reported with variable frequency, between 9% and 36% [19, 28–30, 33–36]. In a recent prospective, multicenter study involving 16 Spanish centers and including 1012 liver transplantations followed up to two years, the incidence of the bloodstream infections (caused by bacteria in 96% of the cases) was 10% [22]. In this study, most bloodstream infection episodes were catheter-related (30%), secondary to surgical site infection (24%) and, to a lesser extent, to pneumonia (6%), whereas the primary source of bacteremia was not identified in 34% of cases. Similar sources of bacteremia have been found by other authors [18, 36,37]. A 7–28% incidence of pulmonary infections has been reported in recent series [17, 19, 21, 28–30, 33,35,38–42]. Other types of infection, such as urinary tract or central nervous system infections, are much less frequently observed [13].
Aside from the general risk factors for infection previously mentioned, other factors specifically favor bacterial infection in liver transplant recipients. Prolonged surgery or reoperation, increased perioperative transfusion, living donor liver transplantation and biliary anastomosis with choledochojejunostomy predispose to surgical site infections [25, 26,43,44]. Specific risk factors for bacterial pneumonia are prolonged mechanical ventilation, persistent non-infectious pulmonary alterations and living donor transplantation [19–20,39]. Rejection and prolonged hospital stay are risk factors for bacterial infections in general [35, 37, 40, 45]. In children, low weight and low age have been associated with increased risk of bacterial infection [30]. The type and intensity of pharmacological immuno-suppression does not clearly influence the risk of bacterial infection, although mTOR inhibitors (sirolimus and everolimus; also called proliferation signal inhibitors, PSIs) may favor surgical-site infections by impairing wound healing [46].
In later post-transplant periods, bacterial infections are much less frequent and are mainly limited to community-acquired infections, especially pneumonia and urinary tract infections [16, 47–49], and to infections related to persisting biliary and arterial complications [32, 33, 50, 51]. Liver transplant patients are slightly more susceptible to some intracellular bacteria, such as Listeria spp., Legionella spp., and Nocardia spp. [47, 52]. The negative impact of late bacterial complications on the survival of liver transplant patients is low or very modest [53–58].
Causative organisms
Organisms causing bacterial infections in liver transplant patients during the early postoperative period correspond to environmental bacteria (transmitted from contaminated persons or equipment) and recipient endogenous bacteria [2, 3, 12, 13, 15, 17, 19–22, 24–28, 34, 36, 45, 59–61], summarized in Table 43.2.
Occasionally, other pathogens, such as Legionella spp., can transiently acquire special relevance [62–64]. Anaerobes are a rare cause of infection in liver transplant patients [35, 29, 30, 61], although Clostridium difficile colitis has been reported as being relatively prevalent in some centers [2, 43]. A substantial proportion of surgical-site and, to a lesser extent, pulmonary infections are polymicrobial. In pneumonia, the isolation of different organisms can correspond to either true polymicrobial infections or subsequent super-infections by pathogens resistant to the current antibiotic therapy [17,20]. As expected, causative organisms in pneumonia cannot be identified in a significant percentage of cases because the microbiological diagnosis frequently requires bronchoscopy with bronchoalveolar lavage, which is not always performed before starting antibiotic therapy [20, 38, 40, 62].
Table 43.2 The commonest microorganisms responsible for major bacterial infections in the early postoperative period following liver transplantation. Only data from recent studies (published from 2000 onwards) are included [17, 19 – 22, 24 – 26, 28, 34, 36, 59 – 61] .
a In some institutions, Acinetobacter baumanii is also very prevalent.
CN: coagulase – negative.
In late periods of liver transplantation, the most frequent bacterial complications are community-acquired pneumonia, mainly caused by Streptococcus. pneumoniae ,Haemophilus influenza and Mycoplasma, and urinary tract infections, mainly caused by Gram-negative bacilli [47, 13, 35, 49]. Other late bacterial infections are cholangitis related to persistent biliary complications, mainly caused by enterobac-teria and Pseudomonas spp. [33], and hepatic abscess secondary to vascular complications, caused by a variety of organisms including Gram-positive cocci, Gram-negative bacilli and anaerobes [51]. Liver transplant patients can also develop tuberculosis. However, since this complication has special characteristics, its prevention and treatment will be addressed separately at the end of this section.
Multi-drug resistant bacteria
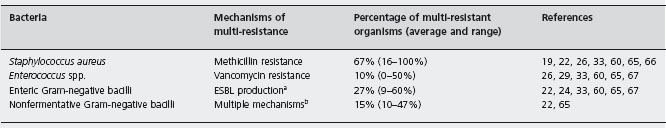
A world-wide problem during the last decades has been the progressive emergence of bacteria resistant to multiple antibiotics. The most relevant multi-resistant bacteria in liver transplant patients are shown in Table 43.3 [19, 22, 24, 26,29,33,59,60,65–67].
Methicillin-resistant Staphylococcus aureus (MRSA) is a frequent cause of early postoperative infection following liver transplantation. Nasal carriage of MRSA (acquired pre- or postoperatively) is associated with an increased risk of MRSA infection [68–72]. MRSA strains are usually susceptible to the glycopeptide antibiotics vancomycin and teicoplanin.
The prevalence of vancomycin-resistant enterococci (VRE) follows a variable geographical distribution, being high in most centers from the USA [65, 71, 73], and low in most European countries [29, 74, 75]. Bowel colonization, acquired pre- or post-transplant, is the major source of VRE infection [71, 76]. Linezolid and daptomycin are active against most VRE [77, 78].
Among the enteric Gram-negative bacilli, Escherichia coli and Klebsiella pneumoniae are the organisms most commonly producing extended-spectrum beta-lactamase (ESBL), although other enterobacteriaceae strains can also express ESBL [79]. In general, ESBL-producing enterobac-teria are susceptible to carbapenems (imipenem, mero-penem and ertapenem) [80, 81].
Pseudomonas spp. are the most relevant multi-resistant non-fermentative Gram-negative bacilli in liver transplant patients. Other multi-resistant members of this family with increasing importance for liver transplant patients are Acinetobacter baumanii and Stenotrophomonas maltophilia. Tigecycline is habitually active against A. baumanii and S. maltophilia [82]. S. maltophilia is also highly susceptible to cotrimoxazole [83].
Several authors have found a direct association between mortality and VRE colonization and infection by some multi-resistant organisms [22, 65, 71, 76].
Prevention
In theory, infection can be prevented by strategies reducing risk factors for infection and those reducing the infective capacity of potentially pathogenic organisms. However, risk factors for infection are either not susceptible to improvement (i.e. the severity of pre-transplant liver disease) or have experienced little change over the last years. Only two surgical and anesthetic approaches should be mentioned. One is the attempt to decrease the operative hemorrhage, an important risk factor for early bacterial complications, by the use of temporary portocaval shunting [84], the reduction of both central venous pressure and plasma transfusion [85], or the administration of aprotinin [86]. The other is the immediate tracheal extubation after surgery (“fast track” policy) to reduce the risk for pneumonia [87]. Nevertheless, the impact of these strategies on the incidence of bacterial infection is uncertain. At present, therefore, prevention of bacterial complications in liver transplant patients is almost exclusively based on interventions on the microbial flora.
aESBL (extended-spectrum beta-lactamase) producing organisms resistant to penicillins, cephalosporins and aztreonam.
bPseudomonas spp. (the most representative organisms of this group) resistant to at least three of the following antibiotics: beta-lactams, carbapenems, aminoglycosides, or quinolones. Other relevant pathogens are Acinetobacter baumanii and Stenotrophomonas maltophilia.
Peri-operative antibiotic prophylaxis
Although controlled studies on this topic are lacking (namely, prophylaxis versus no prophylaxis), peri-operative antibiotic prophylaxis is the standard practice to prevent surgical-site infections as well as other bacterial infections during the very early post-operative period. However, due to the marked differences from one center to another regarding the predominant causative organisms, no specific prophylaxis can be universally recommended. Furthermore, the paucity of prospective, comparative studies on different antibiotic regimens adds important difficulties. The only exception is a study comparing cefazolin and amoxicillin-clavulanate, in which no difference in the rate of surgical-site infection was observed between the two regimes [24]. Unfortunately, these two antibiotic regimes were suboptimal because around 40% of isolated organisms were resistant to the administered agents. A1d Thus, only general rules for peri-operative antibacterial prophylaxis can be made.
The appropriate antibiotic agents must be selected according to the local microbial epidemiology and the susceptibility of the most prevalent organisms at each center [88]. The most currently used antibiotics are third and fourth generation cephalosporins, frequently administered in association with other agents, especially those active against Enterococcus spp., such as ampicillin or amoxicillin (with or without clavulanate) [20, 25, 33, 65, 70, 75, 89, 90]. Other antibiotic combinations involve quinolones, antip-seudomonal penicillins, or aztreonam [25]. One dilemma is the prophylactic use of antibiotics active against multi-resistant bacteria, because it seems judicious to reserve these often last-line antimicrobial agents for treatment [91]. In this setting, only the prophylactic administration of van-comycin or teicoplanin could be reasonable in centers with a high prevalence of MRSA [70, 91–93]. In contrast, the prophylactic administration of antibiotics active against VRE, such as linezolid, or ESBL-producing enterobacteria, such as carbepenems, is uncommon even at institutions with a high prevalence of these microorganisms.
Peri-operative antibiotic prophylaxis is routinely initiated immediately prior to transplantation and prolonged throughout the operation, although in some centers prophylaxis is extended to the first postoperative 24 hours or longer [33, 65, 70, 88, 89, 93].
Although the risk of donor-derived bacterial infection is low and the impact on patient and graft survival is usually negligible, the antibiotic prophylaxis in the recipient is usually adapted to the organisms responsible for the donor infection and its duration prolonged for five to ten days [17,94–96].
Selective bowel decontamination and administration of probiotics and prebiotics
Since a high proportion of early infections are caused by bacteria of intestinal origin, selective decontamination of the digestive tract with nonabsorbable antibiotics has been used in different centers. However, few randomized, prospective, controlled trials have properly assessed the efficacy of this strategy [97–101]. Decontaminating regimens in these studies included gentamicin or tobramycin, poly-myxin or colistin, and amphotericin or nystatin, administered orally for a variable period of time. In one study an oral paste with antimicrobial agents was also administered [100]. A reduction of infections caused by Gram-negative bacilli was attained in most treated groups, although the global rate of early bacterial infection was not significantly different, as emphasized in a recent meta-analysis [102]. A1a Survival was not influenced by the use of selective digestive decontamination. Therefore, the potential benefit of this type of prophylaxis is uncertain and it cannot be routinely recommended [45]. A1a
In two randomized trials, a marked reduction was achieved in the incidence of early infection caused by enteric bacteria with the prophylactic administration of a combination of living lactic acid bacteria (probiotics) and bioactive fibers (prebiotics), but clinical outcomes were not significantly different [103,104]. A1d
Prophylactic measures for multi-drug resistant bacteria
General measures include hand hygiene and barrier precautions, which are applicable for all these organisms [45, 105, 106]. Based on the association of nasal carriage of MRSA with infection by this organism [68–72], periodical pre- and post-operative screening for MRSA and nares decolonization with mupirocin application have been proposed in liver transplant candidates and recipients [69–71, 107]. Unfortunately, one study showed that this policy was not effective as it did not prevent postoperative MRSA infection and was followed by a frequent recurrence of nasal MRSA colonization [108]. B4 Since other sites of colonization may be important, skin cleansing with chlorhexi-dine has also been postulated for a better control of MRSA [109,110], as well as for VRE [111], but no study has evaluated these measures in liver transplant patients.
Antibiotic prophylaxis for invasive procedures
The diagnosis and treatment of biliary complications, especially stenosis, frequently require endoscopic cholangiog-raphy. Bactobilia, that is, the bacterial colonization of bile, has been found with a very high incidence in liver transplant patients with biliary stenosis, stents or previous papillotomy [112]. B4 On the other hand, in a large series of patients undergoing ERCP, the only factor predisposing to cholangitis was liver transplantation [113]. Based on these data, antibiotic prophylaxis for ERCP is recommended in liver transplant patients [114]. Piperacillin-tazobactam and impenem provide adequate coverage [112]. Although, to the knowledge of the authors of this chapter, there are no studies on the risk of cholangitis in patients undergoing percutaneous cholangiography, it seems advisable to follow the same recommendation as for ERCP.
In one study, liver biopsy was associated with sepsis when this procedure was performed in liver transplant patients with choledochojejunostomy [115]. For the authors of this study, antibiotic prophylaxis at the time of liver biopsy may be appropriate in this high-risk subgroup of patients, but biliary obstruction was not categorically excluded at the time of biopsy.
For surgical re-intervention very early after transplantation, the same peri-operative antibiotic prophylaxis as for liver transplantation appears adequate. For surgical interventions in later periods, the general guidelines for non-transplant patients undergoing surgery [116] seem sufficient, although the type of antibiotics to be administered should be adapted to the liver transplant patient condition (i.e. grade of immunosuppression) and local microbial epidemiology. B4
Treatment
Antibiotic treatment should be started whenever a liver transplant patient develops either signs strongly suggesting infection (mainly significant fever), signs of sepsis or a well documented localized infection. Nevertheless, it is important to note that not all febrile episodes in liver transplant recipients correspond to infection [18]. When the need for treatment is defined, and after blood and other relevant site cultures are taken, antibiotic therapy is empirically initiated, without knowledge of the causative organisms and their in vitro susceptibility. Empirical therapy should be selected on the basis of different factors, particularly the site of the suspected infection and the local predominant pathogens and their susceptibility patterns. Other important factors are the time since transplantation, recent antibiotic administration, and the colonization status of the patient, if known. At each institution, protocols for empirical treatment of bacterial complications should be periodically revised and adapted to the changes in epidemiology and in vitro susceptibility of predominant organisms.
According to the commonest pathogens during the early period after transplantation (see Table 43.2), the most frequently used agents in the empiric therapy are third and fourth-generation cephalosporins, piperacilli-tazo-bactam and quinolones [13, 117–119]. The administration of glycopeptides (vancomycin or teicoplanin) and carbap-enems (imipenem, meropenem or ertapenem) can be justified in patients with sepsis and hospitalized in centers with a high incidence of MRSA and ESLB-producing Gram-negative bacilli, respectively [66,118]. Once the responsible bacteria are identified and susceptibility tests are available, further adjustments may be required, consisting of either the withdrawal of superfluous antibiotics or the adequate antimicrobial changes if resistant organisms are isolated. Information on the adequacy of the initial empirical treatment is scarce in liver transplant patients with bacterial infection, although some authors have reported around 20% of failure [20, 61, 117]. However, the effectiveness of the initial empiric therapy does not seem to influence the final outcome provided the antibiotic therapy is rapidly modified according to the microbiological results, as also described in non-transplant populations [120–122]. B4
In later periods after transplantation, community-acquired pnemonia and urinary tract infection, the commonest bacterial infections in this postoperative phase [16, 47–49], are treated according to standard protocols in each center for immunosuppressed patients. Specific antibiotic therapy is administered for infections caused by infrequent but characteristic organisms, such as Listeria spp. and Nocardia spp. [47, 52].
Special considerations
Surgical or percutaneous drainage is often required for the treatment of infections arising in devitalized anatomic sites, such as infected abdominal cavity collections or hepatic abscess [32, 36, 51]. Treatment of cholangitis secondary to biliary stenosis must include the endoscopic, radiological or surgical resolution of the stenosis [50]. The removal of IV lines is mandatory in catheter-related bacteremia [66].
Antibiotic treatment of pneumonia, especially in early stages following liver transplantation and in patients with mechanical ventilation, can be difficult because the responsible organisms are not identified in approximately 50% of episodes [20, 38]. Polymicrobial pneumonia, caused by more than one bacteria or bacteria combined with non-bacterial microbes, such as fungi, cytomegalovirus or P. jirovecii may represent an added difficulty for treatment [17, 20]. Although the empiric treatment of pneumonia attains a positive response in a relatively high proportion of patients, in patients who impair regardless of this therapy, invasive diagnostic procedures, such as fiberoptic bronchoscopy with bronchoalvelar lavage or lung biopsy, must be performed [20, 38, 62]. B4 In spite of an aggressive diagnostic approach, the etiology of pneumonia can only be established on necropsy basis in some patients.
Since most liver transplant patients receive calcineurin inhibitors (tacrolimus or cyclosporine) as immunosuppressive drugs, the potential capacity of different antibiotics to increase the risk of toxicity of calcineurin inhibitors must be taken into consideration [14]. Aminoglycosides can increase the risk of nephrotoxicity, and they should be avoided if possible [123]. Since imipenem may enhance the risk of seizures, particularly in patients with renal function or neurological impairment [124], the administration of other carbapenems (meropenem or ertapenem) may be more appropriate in patients treated with calcineurin inhibitors. Pharmacokinetic interactions of several antibiotics with calcineurin inhibitors metabolized by the hepatic cytochrome P-450 have been described [125, 126]. Rifampicin and nafcillin induce the metabolism of calcineurin inhibitors, with risk of rejection due to low levels of these immunosuppressants, whereas macrolides reduce their metabolism, with the consequent increased risk of toxicity. In patients who necessarily have to receive these antibiotics, a careful monitoring of blood levels of calcineurin inhibitors is mandatory. Since proliferation signal inhibitors (PSIs, also named mTOR inhibitors: sirolimus and everolimus) are also metabolized by the cytochrome P-450 [127, 128], it is likely that similar drug interactions can also occur.
Tuberculosis
The incidence of tuberculosis in liver transplant recipients is around 1–2% in developed countries and 10–15% in endemic regions, which is much higher than that in the general population in the respective countries [129]. Reactivation of latent tuberculosis is considered the main mechanism for active tuberculosis in transplant recipients, which predominantly occurs within the first postoperative year [130, 131]. Approximately half of the patients have pulmonary disease, whereas the other half present with extrapulmonary or disseminated tuberculosis [130, 131]. Aggressive investigations are often required for the diagnosis, and, in some patients, the definitive diagnosis can only be established after a positive response to empiric treatment.
Prevention
In patients receiving kidney transplantation, and, by extension, in patients with extra-renal transplantation, there is the general recommendation to administer antituberculous chemoprophylaxis with isoniazid for the first 9–12 postoperative months in transplant recipients with a past history of tuberculosis and/or pre-operative positive tuberculin test [88, 132–134]. B4 However, regardless of the excellent efficacy of this strategy [130], prophylaxis with isoniazid in liver transplant patients with a positive tuberculin test is challenging for two reasons. First, a positive skin test without other additional risk factors is rarely associated with latent infection reactivation [88]. As an example, in a study by our group, none of 73 patients with a pre-trans-plant positive tuberculin test developed post-transplant tuberculosis regardless of not receiving isoniazid prophylaxis [135]. In contrast, in the same study, tuberculosis was diagnosed in 2% of 279 patients with a negative tuberculin test; the incidence was especially high (10%) in patients with a past history of tuberculosis and untreated with isoniazid prophylaxis, thus suggesting that anergic patients with possible latent tuberculosis were very prone to develop latent infection reactivation. Second, an important concern for generalized isoniazid prophylaxis in liver transplant recipients with a pre-transplant tuberculin skin positive test is the high incidence of isoniazid hepatotoxic-ity, ranging from 17% to 41%, which can outweigh the possible benefit of this strategy [130,135]. For these reasons, although there are no controlled studies on this issue, the general feeling is to limit isoniazid prophylaxis to patients with a history or chest X-ray evidence of past tuberculosis, particularly if it has been inadequately treated, or to patients with a positive skin test and additional risk factors (recent tuberculin conversion, non-Caucasian ethnicity or regional endemicity, and excessive immunosuppression) [88,134]. B4 In the remaining cases, surveillance and treatment of active tuberculosis, if it develops, seem sufficient. Alternative prophylactic regimes, particularly the combination of a quinolone and ethambutol, can be administered in the case of previous isoniazid administration forming part of inadequate antituberculous therapies or in the case of liver graft alterations precluding isoniazid treatment or making it hazardous. Another possibility is to administer prophylaxis while patients are on the waiting list for liver transplantation, although this strategy has been rarely explored [136].
Treatment
The most recommended standard treatment for active tuberculosis in the general population is the combination of rifampicin, isoniazid, pyrazinamid and ethambutol for two months, followed by an additional four-month therapy with rifampicin and isoniazid [137, 138]. In transplant recipients, the length of treatment is extended up to 12 months in pulmonary disease and up to 18 months in extra-pulmonary disease [139–143]. B4 However, this therapeutic regime cannot be accomplished in a substantial proportion of patients due to hepatotoxicity, with an average incidence of around 30–40% [131,130,141,144]. In patients with hepatoxicity or significant liver graft alterations, the most hepatotoxic combination of rifampicin with isoniazid and/or pyrazinamide is usually withdrawn or avoided, and non-hepatotoxic or less hepatotoxic regimes, including ethambutol, quinolones, and rifabutin combined with isoniazid or pyrazinamide, are administered with satisfactory results [140,142,145].
Viral infections
Cytomegalovirus
Cytomegalovirus (CMV) belongs to the human herpesvi-rus family and is a significant opportunistic pathogen in liver transplantation. Infection by CMV is defined as the isolation of CMV or the detection of viral proteins or nuclear acids in whatever fluid or tissue of the organism [146,147].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree