MESENTERIC ISCHEMIA
ESSENTIAL CONCEPTS
Acute mesenteric ischemia (AMI) is a medical and surgical emergency. Delay in diagnosis is associated with high mortality.
Patients often present with abdominal pain out of proportion to physical examination findings.
Clinical suspicion of AMI necessitates early radiologic evaluation (computed tomographic [CT] angiography, conventional angiography) or exploratory surgery in patients with peritoneal signs.
Chronic intestinal ischemia is a clinical diagnosis. Patients report classic symptoms (eg, abdominal angina) and have radiographic findings showing severe stenoses or occlusion of two or more mesenteric arteries.
Colonic ischemia is rarely life threatening and usually resolves with supportive care.
Intestinal ischemia is caused by a reduction in intestinal blood flow, most commonly as a result of occlusion, vasospasm, or hypoperfusion of the mesenteric circulation. It is categorized as acute or chronic, depending on the rapidity and the extent to which blood flow is compromised and whether it is episodic or constant, as might occur in chronic mesenteric ischemia. Ischemia can involve the small intestine or colon. Acute small intestinal ischemia is a medical and surgical emergency that requires prompt diagnosis and a coordinated, interdisciplinary approach. By contrast, acute colonic ischemia (ie, ischemic colitis) is rarely an emergent condition. Acute intestinal ischemia can be further categorized as arterial versus venous, embolic versus thrombotic, and occlusive versus nonocclusive. Other causes of bowel ischemia include strangulating obstructions (adhesions, hernias, metastatic malignancy, intussusceptions) and vasculitis (systemic lupus erythematosus, polyarteritis nodosa).
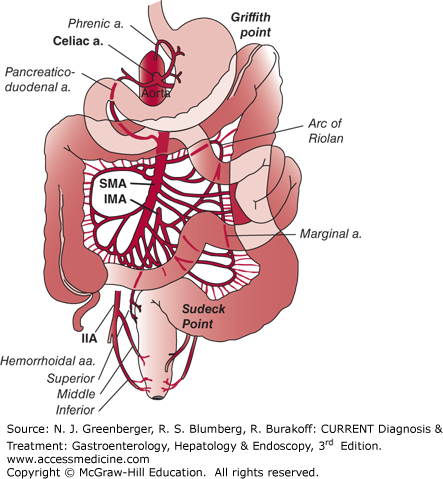
The vascular supply to the intestines includes the celiac artery, the superior mesenteric artery (SMA), and the inferior mesenteric artery (IMA) (Figure 6–1). The celiac axis supplies blood to the stomach and duodenum. The SMA supplies the small bowel from the distal duodenum to the mid-transverse colon. The inferior mesenteric artery supplies the transverse colon to the rectum. Natural anastomoses exist between branches of the major vessels, and if one artery is occluded, some flow may be maintained via a patent collateral vessel.
When a major vessel is occluded, collateral pathways open immediately in response to a fall in arterial pressure distal to the obstruction. The superior and inferior pancreaticoduodenal vessels are collaterals that connect the celiac axis to the SMA. The phrenic artery connects the aorta to the celiac axis. The marginal artery of Drummond and the arc of Riolan are collaterals that connect the SMA and the IMA. The internal iliac arteries provide collaterals to the rectum. Griffith point in the splenic flexure and Sudeck point in the rectosigmoid area are watershed areas within the colonic blood supply and common locations for ischemia.
The splanchnic circulation receives 25% of the cardiac output under basal conditions and 35% or more postprandially. Approximately 70% of splanchnic inflow goes to the mucosa, which is the most metabolically active area of the gut. The villus tips are the most vulnerable to ischemic injury. Intestinal blood flow is under complex regulation controlled primarily by resistance arterioles and precapillary sphincters. Several vasoactive substances influence intestinal perfusion. Catecholamines, angiotensin II, and vasopressin all can cause vasoconstriction, whereas vasoactive intestinal peptide causes vasodilation. Products of ischemia such as acidosis, hypoxemia, and hyperkalemia have been shown to cause vasodilation. Ischemic damage is caused by both hypoxia and reperfusion injury.
Figure 6–1.
Distribution of blood supply to the small intestine and colon from the celiac artery, superior mesenteric artery (SMA), inferior mesenteric artery (IMA), and internal iliac artery (IIA). (Reproduced, with permission, from Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. McGraw-Hill, 2008.)
[PubMed: 10784596]
[PubMed: 12944600]
ACUTE MESENTERIC ISCHEMIA
ESSENTIALS OF DIAGNOSIS
Maintain a high index of clinical suspicion.
Consider in patients >50 years who present with sudden onset of severe abdominal pain lasting >2 hours, especially if a history of cardiovascular disease (congestive heart failure, myocardial infarction, arrhythmia) is present.
Consider in patients with abdominal pain out of proportion to physical findings.
Angiography is the diagnostic procedure of choice and is potentially therapeutic.
AMI remains a challenging diagnosis, with a mortality rate exceeding 50%. The incidence of AMI has increased over the past 20 years due to longer life expectancies, increased awareness of ischemic syndromes, and enhanced diagnostic and therapeutic techniques. The various causes of AMI include SMA embolism (50%), SMA thrombosis (15–20%), nonocclusive mesenteric ischemia (NOMI; 20–25%), and mesenteric venous thrombosis (MVT) (5–10%).
Embolism to the SMA is most frequently due to a dislodged thrombus originating from the left atrium, left ventricle, or cardiac valves. One-third of patients have a history of a prior embolic event, and 20% have synchronous emboli. The onset of symptoms is abrupt and dramatic. SMA thrombosis usually occurs at the origin of the vessel, which is frequently an area of severe atherosclerotic narrowing. Acute thrombosis usually occurs in a patient with underlying chronic intestinal ischemia. This process is analogous to plaque rupture in acute coronary syndromes. Approximately 50% of patients have a history of chronic mesenteric ischemia, and the acute event occurs after closure of a collateral vessel.
Patients with AMI present with severe, acute, unremitting abdominal pain strikingly out of proportion to the initial physical findings. On examination, the abdomen may be soft and either nontender or minimally tender. Distention is often the first sign. Later findings may include signs of peritonitis, especially if infarction or gangrene has occurred. Associated symptoms may include nausea, emesis, and transient diarrhea due to urgent bowel evacuation. Occult blood is found in the stool in 50–75% of cases.
Leukocytosis with a white blood cell count greater than 15,000/μL is found in 75% of patients. However, most patients with early intestinal ischemia do not have abnormal laboratory findings. Lactic acidosis, hemoconcentration, and raised serum aminotransferase levels are usually late findings indicative of intestinal infarction.
Many investigators have searched for the optimal plasma or serum biomarker to easily identify acute mesenteric ischemia. Intestinal fatty acid–binding protein (iFABP) levels, glutathione S-transferase, D-lactate, D-dimer have shown promising and some conflicting results in studies. No single biomarker has achieved sufficient sensitivity, specificity, or accuracy for routine clinical use to diagnose or exclude acute mesenteric ischemia.
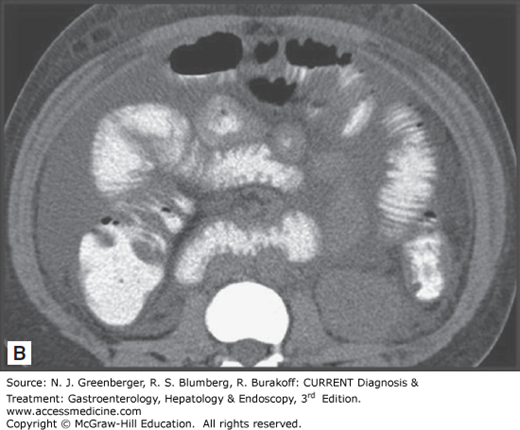
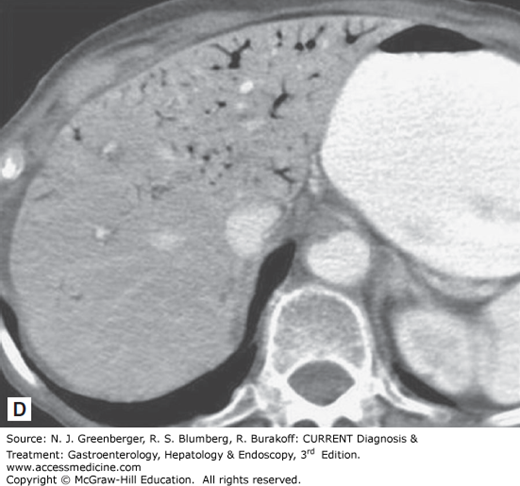
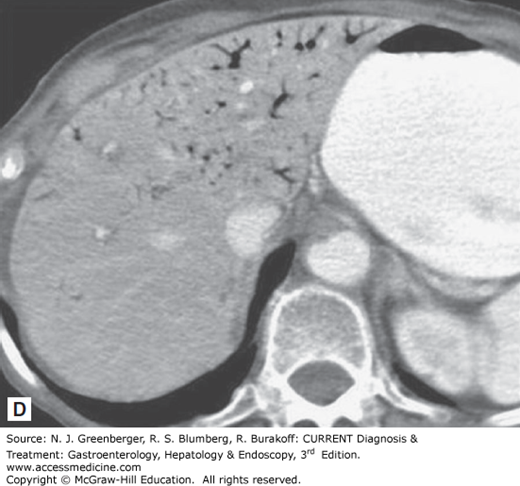
Historically, standard radiologic imaging has not been useful in diagnosing AMI, because most of the reported “classic” findings (eg, bowel edema, pneumatosis intestinalis, portal venous gas) are not seen until late in the course of the illness. Plain films of the abdomen are usually normal, and the utility of these studies is to exclude other acute abdominal processes such as perforation or obstruction. Mesenteric angiography has been the gold standard for making the diagnosis of AMI; however, studies have demonstrated that CT scans may have an overall sensitivity of 80% for AMI, thanks to technical advances, including spiral CT, rapid intravenous bolus injection of contrast, and multidetector-row CT (MDCT) with three-dimensional reconstruction. CT findings may be either more specific or nonspecific. More specific findings include thromboembolism in mesenteric vessels, portal venous gas, bowel wall intramural gas or pneumatosis, lack of bowel wall enhancement, and signs of ischemia in other organs. Less specific signs include diffuse bowel wall thickening, striking vascular engorgement, dilated fluid-filled loops of bowel, and mesenteric edema (Figure 6–2). Magnetic resonance angiography (MRA) can produce similar images; however, image acquisition takes longer than for MDCT, which limits its use in the setting of an acutely ill patient.
Initial evaluation should include radiographic imaging to exclude other causes of acute abdominal pain such as perforation or obstruction. MDCT can provide detailed information about the mesenteric vessels and small bowel and is particularly sensitive in the diagnosis of mesenteric venous thrombosis.
Angiography is important in both diagnosis and management of AMI and remains the gold standard for evaluation of patients with suspected AMI and no peritoneal signs. It is the mainstay of diagnosis and treatment for both occlusive mesenteric ischemia and NOMI. It can be both diagnostic and therapeutic. Placement of a catheter into the SMA allows visualization of obstructing lesions and facilitates interventions such as infusion of vasodilators into the SMA, angioplasty, stenting, or infusion of thrombolytics (Figure 6–3).
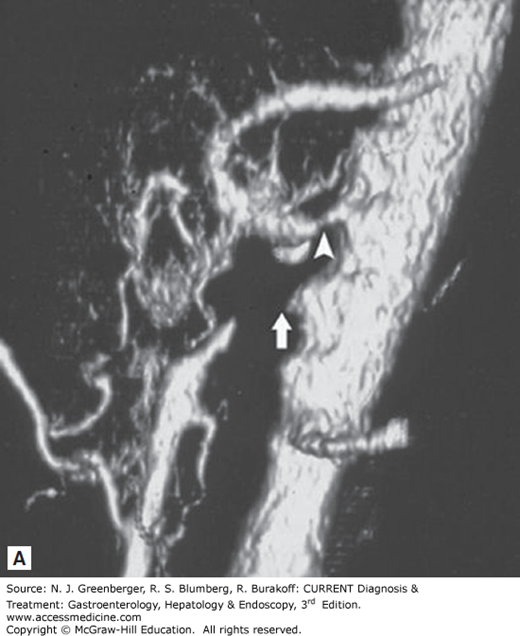
Figure 6–3.
A. Volume-rendered three-dimensional CT scan showing narrowing of the celiac trunk (arrowhead) and occlusion of the proximal SMA (arrow). B. Conventional angiogram showing SMA occlusion (arrow). (Reproduced, with permission, from Kirkpatrick Kroeker MA, Greenberg HM. Biphasic CT with mesenteric CT angiography is the evaluation of acute mesenteric ischemia: initial experience. Radiology. 2003 Oct;229(1):91–98.)
The goal of treatment in AMI is to restore intestinal blood flow as rapidly as possible. However, initial management must include hemodynamic resuscitation and correction of precipitating causes of AMI such as arrhythmias, congestive heart failure, or volume depletion. Patients require aggressive hemodynamic monitoring and support, correction of fluid and electrolyte abnormalities, and treatment with broad-spectrum antibiotics.
Patients with peritoneal signs or clinical suspicion of perforation or gangrene require emergent laparotomy, after hemodynamic stabilization, to immediately restore mesenteric blood flow and resect nonviable bowel. Patients who are hemodynamically stable with no peritoneal signs should undergo angiography to diagnose obstructive lesions and begin treatment with vasodilators, such as papaverine, which when infused directly into mesenteric vessels can help increase blood flow by reversing vasospasm. Once an obstructing lesion is confirmed with angiography, patients can undergo either surgical revascularization (through aortomesenteric bypass grafting, embolectomy, or thromboendarterectomy) or endovascular revascularization (using techniques such as balloon dilation and angioplasty with or without stenting, or catheter-directed thrombolytic therapy, in selected cases). The American Gastroenterological Association algorithm for diagnosis and management of acute intestinal ischemia is presented in Figure 6–4.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree