Fig. 11.1
Scintigraphic image showing parietal cell burden, the acid pocket, in the cardia in a healthy individual. Reprinted with permission from Beaumont H, Bennink RJ, de Jong J, Boeckxstaens GE. The position of the acid pocket as a major risk factor for acidic reflux in healthy subjects and patients with GORD. Gut. 2010;59(4):441–51 [47]. © BMJ Publishing Group
An additional finding from Mason and his colleagues was that gastrin levels are low in most patients after gastric bypass [23]. This low gastrin level would correlate well with the decreased gastric acid stimulation in patients following LRYGB. For those who continue to have low pH within the gastric pouch, it is possible that the acid production occurs from vagal stimulation. Again, the acid production, whether from gastrin or vagal innervation, would respond to proton pump inhibition with medications [14].
As the gastric bypass was slowly being developed, several surgeons created the gastric pouch simply by stapling the stomach and not transecting the pouch from the remainder of the stomach [28]. While eliminating a potential location for leak, this led to an increased incidence of gastrogastric fistula creation [29]. Capella and Capella found that gastric pouches left stapled in continuity or only partially transected led to a gastrogastric fistula rate of 49 % in 189 patients. When they transitioned to complete division of the pouch from the remnant stomach, the rate dropped to 2.6 % in the next 188 patients. Despite this decrease in gastrogastric fistula rate, the incidence of marginal ulceration between the two groups did not change significantly. This is in contrast to Carrodeguas and colleagues who had an incidence of marginal ulceration of 4.2 % within their series of 1292 LRYGB patients. They divided the gastric pouch from the gastric remnant. In the 15 patients (1.2 %) who developed gastrogastric fistulas, the rate of marginal ulceration was 53.3 % [30]. Patel et al. also found an increased incidence of MUs in patients with gastrogastric fistulas. In 2282 patients who had undergone RYGB, a mixture of open and laparoscopic procedures, 122 patients (5.3 %) were found to develop MUs. Of those patients, 28 (22.9 %) were found to have gastrogastric fistulas [31]. Increased jejunal mucosal acid exposure due to the gastrogastric fistula likely increases the risk of marginal ulceration.
Much of the variability in techniques of LRYGB exists in the creation of the gastrojejunal anastomosis. Described techniques include a stapled anastomosis, hand-sewn anastomosis, sewn anastomosis with nonabsorbable suture, sewn anastomosis with absorbable suture [28]. Some of these techniques may predispose LRYGB patients to marginal ulceration. Capella and Capella transitioned from stapled gastrojejunostomies to hand-sewn gastrojejunostomies after they adopted division of the gastric pouch from the gastric remnant [29]. In the stapled gastrojejunostomies, the rate of marginal ulceration remained similar to the rate of ulceration prior to division of the pouch, 5.1 %. Once they began to complete the anastomoses with silk suture, only 5 patients (1.6 %) in 306 developed MUs. They went one step further and switched to absorbable suture from silk. In the next 97 patients with absorbable sutures, no symptomatic marginal ulceration had occurred with follow-up of 10 months.
Sacks and colleagues similarly modified their anastomotic technique. After using a linear stapler to create the gastrojejunostomy, the common gastroenterotomy was closed using an inner layer of running nonabsorbable suture followed by a second seromuscular layer of nonabsorbable suture. They transitioned to an inner layer of absorbable suture [32]. With this change, the rate of MUs decreased from 2.6 %, 28 of 1095 LRYGB patients, to 1.3 %, 29 of 2190 LRYGB patients (Fig. 11.2).
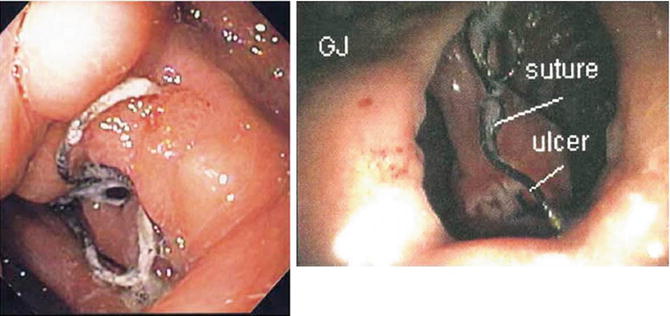

Fig. 11.2
Marginal ulcer seen on endoscopy with suture material within the ulcer base; GJ = gastrojejunostomy. Reprinted with permission from Sacks BC, Mattar SG, Qureshi FG, Eid GM, Collins JL, Barinas-Mitchell EJ, Schauer PR, Ramanathan RC. Incidence of marginal ulcers and the use of absorbable anastomotic sutures in laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2(1):11–6 [32]. © Elsevier
Suture used to reinforce stapled gastrojejunal anastomoses may also predispose LRYGB patients to the development of MUs. Vasquez et al. transitioned from an outer reinforcing suture layer of nonabsorbable suture to absorbable suture. Both groups had a 25-mm circular stapled gastrojejunal anastomosis. Use of absorbable suture had fewer MUs, 2 patients of 84 LRYGB patients (2.3 %), versus 31 patients out of 231 (13.4 %) with nonabsorbable suture [33].
Along with variations in techniques for creating the gastrojejunostomy, two variations exist for positioning the Roux limb during a LRYGB. Both antecolic and retrocolic Roux limb positions are used routinely. The debate over the best position continues, mostly around the incidence of internal hernias. The variation may also contribute to a higher incidence of MUs. Ribeiro-Parenti and her colleagues examined two large cohorts of patients using the antecolic position first, followed by the retrocolic technique, with 572 and 570 consecutive patients, respectively [34]. In their 1142 patients, 46 patients developed symptomatic MUs (4 %). Patients in the antecolic group were more likely to develop MUs and more likely to develop those ulcers early, within 3 months of surgery. Also, 2 patients with MUs in the antecolic group went on to perforate while no patients in the retrocolic group did. The two cohorts were comparable in regards to other risks for MUs, such as tobacco use and NSAIDs. The difference in MU rates between the two Roux positions may be due to more tension on the anastomosis, with an antecolic Roux limb possibly resulting in mucosal ischemia.
11.5 Diagnosis
The diagnosis of MUs, as with all complications, requires a high level of suspicion. LRYGB patients who present with new onset epigastric pain should be considered for MUs. However, internal hernias, stenosis, adhesive bowel obstructions, biliary disease, or cardiac disease may all present with epigastric pain. For those MUs that present with nausea or vomiting; internal hernias, stenosis, adhesive bowel obstructions and biliary disease must again be considered. If bleeding is the initial symptom, then bleeding from the large bowel may also require evaluation. With a long differential diagnosis accompanying the usual presentation of epigastric pain, nausea, vomiting, or bleeding, a systematic and careful evaluation is required [9] (Fig. 11.3). For patients that do not present acutely and develop insidious symptoms, the bariatric support staff are important in helping to make the diagnosis of MU. Registered dieticians and primary care providers will see patients more postoperatively than surgeons to assist in dietary changes and alterations to medication regimens. As such, bariatric surgeons should ensure that those working with their patients are aware of the complications and the need to report these symptoms for further evaluation [35].
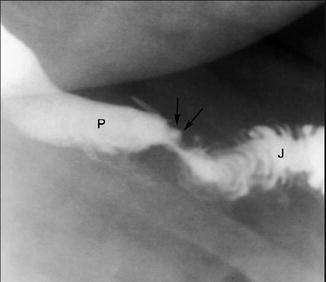

Fig. 11.4
Upper gastrointestinal fluoroscopic spot image with oral contrast showing retained contrast within the ulcer base (arrows). Patient is in the left posterior oblique position; P = pouch, J = jejunum. Reprinted with permission from Carucci LR, Turner MA. Radiologic evaluation following Roux-en-Y gastric bypass surgery for morbid obesity. Eur J Radiol. 2005;53(3):353–65 [36]. © Elsevier
For MUs presenting acutely, such as with perforation, resuscitation and possibly emergent surgery may usurp any other diagnostics. For patients who are stable with suspicion of MUs, radiographic evaluation is the next step in evaluation as clinical evaluation in the obese patient is usually difficult. Upper gastrointestinal (UGI) examinations using fluoroscopy can be very helpful in the diagnosis of epigastric abdominal pain, evaluating for leaks or obstruction. MUs may also be seen. They will likely appear as a small focal collection of contrast within the ulcer that remains as the luminal contrast continues distally (Fig. 11.4). It will be located near the gastrojejunal anastomosis, either on the anastomosis or in the jejunum distally. While not completely diagnostic, UGI does serve as an initial first step in the workup of MUs and will help to rule out other diagnoses but may not always be necessary [36].
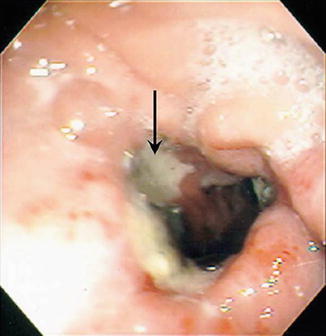

Fig. 11.5
Marginal ulcer (arrow) seen just beyond gastrojejunostomy 1 month following laparoscopic gastric bypass. Reprinted with permission from Gumbs AA, Duffy AJ, Bell RL. Incidence and management of marginal ulceration after laparoscopic Roux-Y gastric bypass. Surg Obes Relat Dis. 2006;2(4):460–3 [41]. © Elsevier
UGI will also assist in the diagnosis of potential causes of MUs. Gastrogastric fistulas can sometimes be seen on UGI as a communication between the gastric pouch and the excluded stomach. Computed tomography (CT) is usually a mainstay of evaluation for postoperative bariatric patients, particularly in the acute care setting within the emergency department. Findings on CT will likely be within normal limits unless oral contrast is given. With oral contrast, the findings will be similar to UGI. Complications of MUs may also be seen with CT. Free air or extravasation of contrast may be seen with perforation. Gastrogastric fistulas may also be seen as contrast traversing from the gastric pouch to the excluded stomach [37]. CT will also help to eliminate other potential causes of epigastric pain, nausea, or vomiting, such as internal hernias or leaks.
After radiographic evaluation that may show findings concerning for MUs or eliminate other potential diagnoses endoscopy remains the gold standard for the diagnosis of MUs. It also serves as a method of treatment. On endoscopy, the ulcer will be visible at the anastomosis or on the jejunal mucosa of the Roux limb (Fig. 11.5). Etiology of the ulcer may also be seen on endoscopy, such as suture material within the ulcer. Histologic and testing of biopsied surrounding mucosa may also help to eliminate H. pylori as a potential etiology.
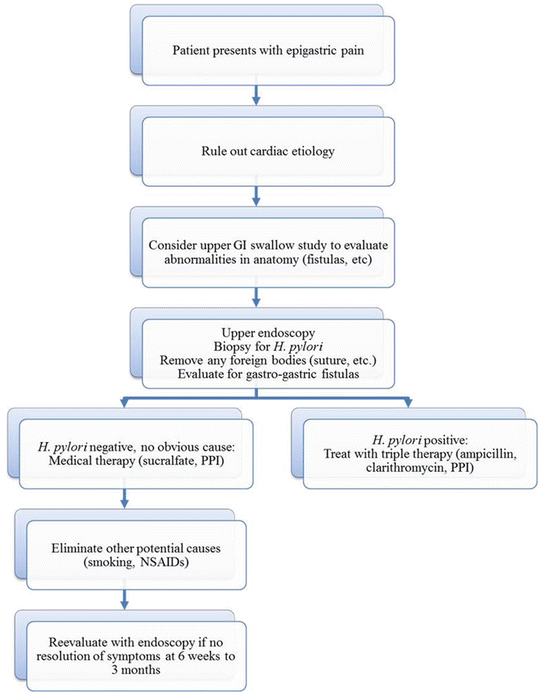

Fig. 11.3
Diagnostic flowchart for marginal ulceration following laparoscopic Roux-en-Y gastric bypass
11.6 Prevention and Management
As with all surgical complications, prevention of the complication is better than eventual treatment. Because of the multiple causes of MUs that have been postulated, strategies for prevention vary. At a minimum, the ASMBS recommends smoking cessation counseling prior to surgery [18]. In our practice, patients must abstain from all tobacco products for 3 months prior to any bariatric surgeries. Many patients who wish to undergo bariatric surgery will present with joint disease and will likely be taking NSAIDs for pain and inflammation management. This presents a difficult situation for health care providers of patients who desire LRYGB. NSAIDs will increase the risk of developing MUs, but without them, the patients may not ambulate as much as is necessary to prevent other complications, such as venous thromboembolism. Attempts should be made to limit the amount of NSAIDs that patients take preoperatively and cessation of NSAID therapy should follow LRYGB as soon as possible. For postoperative LRYGB patients who present with injuries where NSAIDs would be used as treatment, alternative therapies should be attempted.
H. pylori infection in bariatric patients has a reported prevalence of 24–67 % in the literature [38]. While its causal relationship with peptic ulcer disease is well known, its exact relationship with MUs is still debatable. As such, many bariatric programs will screen for H. pylori infection prior to LRYGB. Hartin and colleagues found that not testing for H. pylori did not increase the incidence of MU formation but did confer a greater risk of perforation if the patients went on to develop ulcers [22]. While H. pylori infection may not directly cause ulcers, its presence has been linked to epigastric symptoms following LRYGB. Ramaswamy et al. found that patients were more likely to complain of dyspepsia symptoms without MUs if infected with H. pylori [38]. Based on this, they recommended testing for H. pylori infection as eradication may decrease postoperative foregut symptoms and MU formation. Contrary to this, Papasavas and colleagues found no difference in MU rate [20]. Currently, the ASMBS suggests testing for H. pylori in regions where there is a high prevalence of infection [18]. Most of the USA has a relatively low prevalence. Patients from developing countries who desire LRYGB would likely benefit from preoperative screening for H. pylori. Testing for infection may be completed noninvasively through the urease breath test or stool antigen study. Invasive testing requiring endoscopy requires biopsies and also evaluates for urease or microscopic visualization of the organisms. Patients who present with epigastric symptoms following surgery should also be tested for H. pylori and undergo eradication if positive. The most commonly recommended initial regimen includes a proton pump inhibitor (PPI), amoxicillin, and clarithromycin. Regimens for the eradication of H. pylori have been designed for patients of normal body mass index. Morbidly obese patients may not respond to the same regimen [39]. If eradication is needed, confirmation should be completed.
Intraoperative prevention of ulceration includes ensuring that the gastric pouch is appropriately sized to exclude as much parietal cell mass as possible. The pouch should be divided from the gastric remnant, not just stapled in continuity, to decrease the risk of gastrogastric fistula formation. Absorbable sutures should be used for the inner layer of the gastrojejunal anastomosis, and the Roux limb must be under no tension to prevent any possible ischemia at the anastomosis.
Postoperative medication regimens, such as the use of PPIs for MU prevention, vary by institution. The ASMBS currently has no recommendations about the use of PPIs postoperatively for the prevention of MUs [18]. No conclusive evidence exists for the use of PPIs or H2 blockers, such as ranitidine, postoperatively, except in cases of eradication of H. pylori. One study by D’Hondt and his colleagues showed no difference in LRYGB patients who underwent a short course of omeprazole 20 mg daily for 1 month after surgery than in those who did not. Both formed MUs at the same rate. A difference was seen in patients who had undergone H. pylori eradication prior to surgery. For those patients, use of prophylactic low-dose PPI therapy did decrease the rate of MU formation [40]. Garrido et al. showed this as well after following 118 LRYGB patients who were placed on esomeprazole for 60 days after surgery. Nine patients (7.6 %) still developed MUs despite therapy [19]. With the lack of conclusive evidence, each individual institution and surgeon should implement a plan for postoperative medication use to prevent MUs.
Once a MU has been diagnosed, medical treatment remains the mainstay of treatment. Multiple studies have been completed showing that medication alone will adequately treat the majority of MUs. In a study of 26 patients who developed MUs following LRYGB, all of the MUs resolved following high-dose PPIs [41]. Others recommend use of sucralfate to assist in the healing of the MUs (Fig. 11.6). A suspension of the sucralfate is created using warm water and the large tablets. Theoretically, this will coat the ulcer base, no matter the cause, and assist in healing. No conclusive evidence exists for the use of sucralfate or PPIs or a combined therapy [6]. Sucralfate may help to heal ulcers not associated with acid, as is possible with MUs. When an MU is suspected, beginning high dose PPI for acid suppression with or without the use of sucralfate is the beginning step in treatment and to prevent complications.