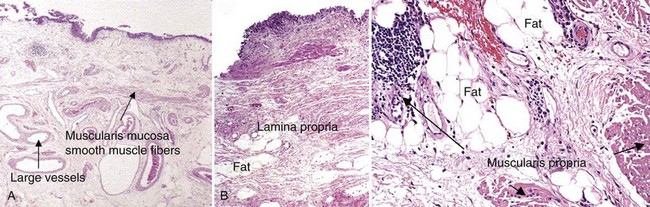
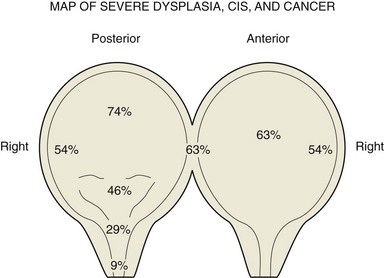
Seth P. Lerner, MD, FACS, Cora N. Sternberg, MD, FACP Primary cancers of the bladder arise from the urothelium, and by far the most common cell type is transitional cell. Squamous cell predominates in Middle Eastern countries where bilharziasis is endemic. It occurs more frequently in Western countries in women and in the setting of chronic indwelling catheters and recurrent infection. As the prevalence of bilharziasis has diminished significantly, transitional cell carcinoma (TCC) now accounts for more than one half of bladder cancer diagnosed in Egypt (el-Mekresh et al, 2009). Adenocarcinomas of the bladder are uncommon but occur in the bladder base/trigone area. Patients with exstrophy are at increased risk. Surgical treatment of urachal adenocarcinoma is partial cystectomy, and the biology is similar to that of colorectal adenocarcinoma. Small cell neuroendocrine cancer is also a rare variant and may be the predominant histology or may be mixed with TCC (Mazzucchelli et al, 2009). This phenotype is easily distinguished by immunohistochemistry expressing synaptophysin and chromogranin. Treatment requires neoadjuvant chemotherapy (cisplatin and etoposide) followed by radical cystectomy or radiation therapy (Siefker-Radtke et al, 2004). Small cell tumors may also be associated with paraneoplastic syndromes including ectopic adrenocorticotropic hormone (ACTH) production, hypercalcemia, and hypophosphatemia. Other neuroendocrine tumors occur in the bladder and include carcinoid tumors, which may originate in the bladder or invade the bladder from the gastrointestinal tract, most commonly from the appendix. Large cell neuroendocrine tumors are rare with few cases reported in the literature and are believed to behave in a manner similar to that of small cell tumors (Akamatsu et al, 2008). Other unusual variants reported have included lymphoepithelioma-like cancers, which have a better prognosis than high-grade invasive TCCs, although treatment is similar stage for stage. The micropapillary variant resembles ovarian papillary serous cancer and is aggressive. It tends toward high grade and high stage and is frequently associated with lymphatic and vascular invasion (Kamat et al, 2006). Radical cystectomy is the treatment of choice because this phenotype typically does not respond to chemotherapy directed at TCC. Rhabdomyosarcoma is seen in both children and adults, and leiomyosarcoma is seen in adults. Primary lymphoma of the bladder is rare, but the bladder may be involved in up to 10% of cases of systemic lymphoma. The 1997 TNM staging schema introduced a modification of prior staging schemes. Before 1997, T2 described disease in the inner half of muscle and T3a described disease in the outer half. T3b was used to identify all levels of perivesical fat invasion. In the current system T2a and T2b are used to designate the inner and outer half of the muscle, respectively, and the T3 category is split into T3a (microscopic) and T3b (macroscopic, or visible on gross inspection of the cystectomy specimen). The T4a prostate designation requires established stromal invasion, which can occur via the urethra or as direct extension via the bladder neck or posteriorly into the seminal vesicles or periprostatic tissue. Finding carcinoma in situ (CIS) in the prostatic urethra or ducts does not lead to upstaging because outcome is determined by the primary bladder cancer stage (Esrig et al, 1996; Pagano et al; 1996). Staging of nodes and metastases is similar to that for other genitourinary malignancies. Positive nodes above the common iliac bifurcation are classified as N+M1 disease. The UICC and AJCC staging systems were uniform in the sixth edition of the American Joint Committee on Cancer (AJCC) Staging Manual (Greene et al, 2002). An updated seventh edition includes the number of nodes removed as a quality measure of radical cystectomy. In addition, node metastases above the common iliac bifurcation are classified as N3 rather than M1 (Table 82–1) (Edge et al, 2010). European Association of Urology (EAU) Guidelines require muscularis propria in the TURBT specimen for accurate staging of invasive cancers. In the absence of muscularis propria, understaging of T1 disease is common, and, unfortunately, this leads to worse outcomes for patients with T1 cancer (Dutta et al, 2001). True, T1 is often mistaken for T2 because there are fine muscle bundles called muscularis mucosa present within the lamina propria (Fig. 82–1A and B). There are blood vessels in this area as well. These muscle bundles may be confused with the muscularis propria and lead to overstaging of a T1 tumor as T2. In addition, recent data suggest that up to 40% of patients initially diagnosed with T1 who undergo cystectomy have pathologic T2 disease (Dutta et al, 2001). Because the management of patients with high-grade T1 tumors is challenging, it is imperative to stage these patients accurately before embarking on intravesical therapy. We therefore routinely re-resect T1G3 tumors before determining appropriate therapy even when we performed the original resection. Presence of persistent T1 cancer on re-resection is a highly significant predictor of poor outcome in patients treated with bacillus Calmette-Guérin (BCG) and is an indication for early cystectomy (Herr et al, 2007). Additional indications for initial cystectomy in patients with T1 disease include size greater than 3 cm, micropapillary histology, and lymphovascular invasion. Fat can be observed in the lamina propria and deep portions of the muscularis propria and should not be confused with perivesical fat invasion (see Fig. 82–1B). In stage T3, cancer appears in fat in the absence of muscle bundles. The goals in staging the primary tumor are to establish histology; look for lymphatic/vascular invasion, which is a risk factor for metastasis; and determine the depth of penetration through a combination of biopsy and bimanual examination. When cystectomy is anticipated, complete resection is not necessary and the risk of bladder perforation is avoided. Downstaging, however, may provide some benefit because retrospective studies and secondary analysis of randomized neoadjuvant chemotherapy trials suggest that survival may be improved when the pathologic tumor stage is less than the precystectomy clinical stage for clinical stage T2 patients (Grossman et al, 2003a; Sonpavde et al, 2009). Patients who are P0 at cystectomy may progress, but at a lower rate than patients with residual invasive cancer (Grossman et al, 2003a). One must also determine whether the local tumor is causing upper tract obstruction and whether there is a concomitant upper tract tumor that also needs treatment. This is accomplished by imaging with computed tomography, magnetic resonance imaging (MRI), and/or retrograde ureteropyelography. With the patient anesthetized, the T stage of the tumor is identified with a combination of transurethral resection and bimanual examination. Findings described originally by Marshall in 1952 are T2a—nonpalpable; T2b—induration but no three-dimensional mass; T3—a three-dimensional mass that is mobile; T4a—invading adjacent structures such as the prostate, vagina, or rectum; T4b—fixed to pelvic sidewall and not mobile (Marshall, 1952). Because tumors of the trigone or those encroaching on the bladder neck in men may directly invade the prostatic stroma, additional tissue should be obtained at resection for accurate staging because this may affect decisions regarding neoadjuvant chemotherapy. The utility of site-directed biopsies or biopsy of normal-appearing, remote mucosa is controversial in non–muscle-invasive cancer (Kiemeney et al, 1994; van der Meijden et al, 1999). However, with high-grade muscle-invasive cancers, at least one half of patients have associated CIS that may affect treatment decisions and outcome if remote from the index tumor (Shariat et al, 2007). The last piece of information needed is the status of the bladder neck (female) or prostatic urethra (male) to serve as a basis for planning appropriate management of the urethra and urinary diversion. Biopsies of the prostatic urethra may provide useful information before radical cystectomy (Lerner et al, 2008a). Using a resectoscope, the surgeon takes a full loop from the midprostate (or bladder neck in shorter prostates) to the mid to distal verumontanum and 5 and 7 o’clock adjacent to the verumontanum. This is the site of the highest concentration of prostatic ducts and the place where CIS is most likely to be found (Sakamoto et al, 1993). The full-thickness chip allows the pathologist to see the interface between the urethral mucosa, prostatic ducts, and stroma. This method is the most accurate means of staging the prostatic urethra (Wood et al, 1989). Negative prostatic urethra biopsies are associated with a negative apical urethral margin and obviate the need for intraoperative frozen section (Lerner and Shen, 2008b). In women, bladder neck biopsies are an accurate surrogate for urethral biopsy when removal of the distal two thirds of the urethra and orthotopic diversion is being considered. Computed tomography (CT) has largely replaced intravenous urography for upper tract imaging. CT provides the added benefit of cross-sectional imaging of the pelvic, iliac, and retroperitoneal lymph nodes, as well as the liver. The incidence of pelvic node metastases is directly related to the depth of invasion of the primary tumor (Table 82–2) (Stein et al, 2001; Leissner et al, 2004; Vazina et al, 2004; Steven and Poulsen, 2007; Ghoneim et al, 2008) and the presence of lymphovascular invasion (Quek et al, 2005). Table 82–2 Incidence of Pathologic Pelvic Node Metastasis (%) at Radical Cystectomy in Selected Contemporary Series (2000-2009) Histologic confirmation of metastatic disease is desirable because its presence has a significant effect on treatment decisions and evaluating response to subsequent therapy. A chest radiograph is important for evaluating the lungs and mediastinum. A chest CT is obtained to further define abnormalities and should be obtained routinely in patients with muscle-invasive disease or N+ disease in whom the risk of visceral metastasis is greatest. Similarly, bone scintigraphy is obtained in these highest-risk patients and in patients with an elevated alkaline phosphatase or new-onset bone pain. An MRI may be useful for patients with an allergy to iodinated contrast or to resolve questions about an equivocal bone scan. The introduction of novel contrast agents with MRI may provide a more sensitive means for detecting nodal metastases. Ferromagnetic nanoparticles, for example, are taken up by macrophages, and dark areas represented by metastatic deposits can be detected with foci as small as 3 mm (Deserno et al, 2004). These studies, which require a 3 Tesla magnet, need further study and validation. Positron emission tomography (PET) is used increasingly in oncology. Combination with CT is now standard and thus provides more accurate localization of abnormalities. Although there is as yet no defined role for routine staging of nodal or visceral metastases in invasive bladder cancer, PET/CT can be particularly helpful in discerning nodal or visceral metastatic disease when these findings will determine the use of chemotherapy or surgery (Kibel et al, 2009). Several commercially available serum biomarkers (CEA, CA-125, CA-19.9) have been reported to be elevated in patients with advanced disease and may provide a marker that correlates with disease activity and response to therapy, but they are not standard (Margel et al, 2006; Kouba et al, 2009). Radical cystectomy provides excellent local control of the primary tumor and should include the bladder and surrounding perivesical soft tissue, prostate, and seminal vesicles in men and the ovaries, uterus/cervix, and anterior vagina in women. In sexually active women, vaginal preservation and/or reconstruction must be discussed and planned preoperatively. Recent reports challenge the dogma of removal of the internal female organs because involvement of the uterus, cervix, and ovaries is uncommon (Chang et al, 2002). Preservation of the vagina and uterus provides better support for a neobladder and the pelvic floor when the extent of the cancer or the age and general health status of the patient does not warrant anterior pelvic exenteration (Ali-El-Dein et al, 2002). Involvement of the urethra or bladder neck is an absolute contraindication to sparing the urethra in women, and a posterior-based invasive cancer is a relative contraindication. Lymph node (LN) metastasis is found in 20% to 25% of patients who undergo radical cystectomy (RC) and pelvic lymphadenectomy for bladder cancer, and it is the most important prognostic factor in these patients, predicting significantly decreased recurrence-free survival and overall survival compared with that for patients without nodal metastases (Lerner et al, 1993; Poulsen et al, 1998; Stein et al, 2001). In node-negative patients, the total number of LNs removed and the anatomic extent of the node dissection are both useful measures in evaluating the proper extent of surgery and predicting outcome. In patients with nodal metastasis, the number of nodes removed and the number and percent of positive nodes may both be independent predictors of recurrence and survival (Leissner et al, 2000; Herr et al, 2002; Herr, 2003; Stein et al, 2003b). The extent of the lymph node dissection as it relates to cancer control and detection of lymph node metastases is an important area for research. The minimum number of lymph nodes to be removed for accurate staging and therapeutic value in bladder cancer has yet to be defined (Mills et al, 2001; Vazina et al, 2004). The unfortunate reality revealed in the Surveillance, Epidemiology and End Results (SEER) database in 2003 was that the majority of patients in a population-based analysis had four or fewer nodes removed with cystectomy (Konety et al, 2003; Hollenbeck et al, 2008). Some improvement had occurred by 2004 with an increase in the number of nodes removed and fewer patients undergoing no node dissection at all (Hellenthal et al, 2009). The wide range of total lymph node dissections reported may reflect a lack of standardization of the extent of node dissection, different sampling techniques, and differences in processing of tissue by the pathologist (en bloc removal vs. presentation in packets). Bochner and colleagues (2001, 2004) demonstrated that simply presenting the nodes to the pathologist in packets rather than en bloc with the cystectomy specimen increased the node count. This has been corroborated by others and should be considered the standard of care (Stein et al, 2007; Ather et al, 2008). Node dissection that includes the common iliac, presacral, and precaval/preaortic nodes may result in a higher number of lymph nodes removed (Vazina et al, 2004). Patient’s age and comorbidities are factors that may affect a surgeon’s decision about the extent of LN dissection, thus influencing the number of LNs retrieved (Bochner et al, 2004; Vazina et al, 2004). A recent study, although small, suggested that in a single institution an individual surgeon had an independent impact on number of nodes removed (Kulkarni et al, 2008). This is not surprising because significant variability in the extent of the node dissection and node counts has been observed in prospective studies requiring a predefined anatomic extent of lymphadenectomy (Leissner et al, 2004; Lerner et al, 2009). Hospital and patient mix affecting comorbidities may affect node counts and mortality associated with cystectomy (Hollenbeck et al, 2008). The current TNM staging system distinguishes between number of nodes with metastases, the size of nodal metastases, and the anatomic location, with nodal metastasis above the common iliac bifurcation denoting M1 disease and thus equivalence to visceral metastasis. The seventh edition of the American Joint Committee on Cancer (AJCC) Staging Manual (released October 2009) notes that at least 12 nodes should be removed. Most studies comparing extended and “standard” nodal dissection or packet presentation of the lymph nodes suggest that the overall incidence of node positivity is not altered, but, in fact, the number of nodes examined may be increased by extending the anatomic extent of the dissection (Poulsen et al, 1998; Vazina et al, 2004; Lerner et al, 2009; Edge et al, 2010). With the focus on number of nodes removed and number of positive nodes as prognostic factors, investigators have developed a combined variable of lymph node density (LND), which takes both of these variables into account. Separate reports from the University of Southern California (USC) and Memorial Sloan-Kettering Cancer Center (MSKCC) published in 2003 described this and determined that LND (positive nodes/total nodes removed) had prognostic significance independent of the prognostic value of the number of positive nodes, pathologic tumor stage, and history of adjuvant chemotherapy (Herr, 2003; Stein et al, 2003b). The prognostic importance of LND has been corroborated in several retrospectively evaluated series (Kassouf et al, 2008; Osawa et al, 2009). An important caveat is that LND is affected by all of the factors described earlier that potentially affect the quality of the node dissection and interpretation by the pathologist (Svatek and Shariat, 2008). Quek and Flanigan wrote an excellent review of this subject and concluded that the body of evidence, based largely on retrospective review, suggests that LN density is an important pathologic prognostic variable (Quek and Flanigan, 2009). As indicated later, prospective validation is important and results lead to improved prognostic models. Koppie and colleagues (2006) reported a study designed to determine if there is a minimum number of lymph nodes analyzed above which there is no improvement in survival. The cohort included 1121 patients from MSKCC accrued over a 14-year period, and the investigators determined that there was no plateau in the dose-response curve with increasing number of nodes up to 23 nodes because few had 24 or more nodes removed. The authors did not indicate the percent of patients who underwent an extended node dissection, and, in fact, 13% had no nodes identified in the pathology report. The median number of nodes removed was 9. Capitanio and colleagues (2009) reported a similar analysis from the Bladder Cancer Research Consortium (BCRC), a three-center database including Baylor College of Medicine, University of Texas Southwestern, and Johns Hopkins University. The median number of nodes removed was 19, and all patients underwent at least a bilateral pelvic node dissection. Using receiver operator curve analysis, the authors determined that analysis of 45 nodes resulted in detection of 90% of patients with nodal metastasis. Removal of 25 nodes resulted in detection of 75% (Fig. 82–2). The largest gain in sensitivity was between 15 and 30 nodes, and there was only modest improvement in sensitivity with examination of more than 30 nodes. They suggested that 25 nodes be the minimum number analyzed, and these findings provide some insight as to why a threshold was not observed in the MSKCC series. (Reprinted with permission from Capitanio U, Suardi N, Shariat SF, et al. Assessing the minimum number of lymph nodes needed at radical cystectomy in patients with bladder cancer. BJU Int 2009;103:1359–62, Fig. 1, p. 1360.) With so many factors contributing to the variability in node counts, one could argue that the anatomic extent and completeness of the node dissection deserve the most emphasis. It is clear that the more tissue removed, the more nodes a diligent pathologist will identify, and this should increase sensitivity in detecting nodal metastases. It is also clear that small-volume nodal disease is compatible with long-term survival, especially when the primary bladder cancer is organ confined. The integration of systemic chemotherapy either before or after radical cystectomy provides an incremental survival benefit, and a thorough node dissection is an integral component of this strategy (Herr et al, 2004). If there is a survival advantage associated with a more extensive node dissection, it is most likely due to the complete removal of all potential node-bearing tissue in the primary and secondary landing zones. This would lead to improved local and regional cancer control and to a more precise identification of patients with nodal metastasis who might benefit from adjuvant chemotherapy. Despite level 1 evidence supporting the use of neoadjuvant chemotherapy for muscle-invasive bladder cancer and a meta-analysis of adjuvant chemotherapy also supporting use of perioperative chemotherapy, recent estimates suggest that only 12% of patients are treated with an integrated treatment strategy of cystectomy and systemic chemotherapy (David et al, 2007). This reality requires that urologic surgeons performing radical cystectomy optimize the anatomic extent and completeness of the lymph node dissection. Skinner and colleagues suggested in 1982 that “a meticulous pelvic node dissection can make a difference,” reporting 5-year survival of 36% in patients with node-positive disease (Skinner, 1982). In addition, they noted excellent local control and no increase in morbidity. These data were further supported in a larger series that also introduced the concept of lymph node density (Stein et al, 2003b). Poulsen and colleagues (1998) suggested that the extended node dissection as described by Skinner and colleagues was associated with better survival for patients with organ-confined cancers and patients with negative nodes than for patients with a pelvic dissection that included only the external and internal iliac and obturator nodes. Recently, Steven and Poulsen confirmed their earlier observation in a consecutive series of 336 patients who had an extended node dissection (Steven and Poulsen, 2007). Patients with lymph node metastases above the common iliac bifurcation had a survival probability similar to that of patients with nodal metastases confined to the pelvis (37% vs. 42%, respectively). An editorial commented that stage migration might account for some of the improvement in survival associated with an extended node dissection, particularly for patients with negative nodes with a more complete node dissection and meticulous pathologic evaluation (Poulsen et al, 1998). The question of whether an extended node dissection improves overall survival or whether that benefit applies only to particular subgroups should be addressed in the context of a randomized clinical trial. Much has been written regarding the utility of intraoperative frozen section (FS) biopsy to identify CIS at the ureteral margin (Schoenberg et al, 1996; Silver et al, 1997; Balaji et al, 1999; Sved et al, 2004; Lee et al, 2006; Raj et al, 2006; Osman et al, 2007). Although previous retrospective studies suggested that FS lacked sufficient correlation with final margin status, contemporary studies both retrospective and prospective found high sensitivity and specificity and positive and negative predictive values for final margin status (Raj et al, 2006; Osman et al, 2007). There is little disagreement about whether every effort should be made to obtain a negative proximal margin before reimplantation when frank tumor is encountered at the margin. It is clear, moreover, that atypia and dysplasia do not require any action. In the case of CIS, an attempt is made to achieve a negative margin without compromising ureteral length because nephrectomy is not indicated for CIS of the ureter. Raj and colleagues, however, question the value of achieving a negative margin because this did not alter the risk of development of subsequent upper tract tumor (Raj et al, 2006). CIS of the ureter is not independently associated with a worse outcome following cystectomy (Lee et al, 2006). Cancer recurrence at the anastomosis is rare even with a positive margin showing CIS, but a positive margin is a risk factor for developing a second primary tumor of the ureter or renal pelvis (Lee et al, 2006; Raj et al, 2006). Upper tract tumors found after cystectomy are often at a more advanced stage and, particularly when symptomatic, may be associated with a worse prognosis (Balaji et al, 1999; Sved et al, 2004; Sanderson et al, 2007). Surveillance ureteroscopy is the most sensitive means for following patients with a positive ureteral margin, and long-term follow-up is required. The median time to occurrence in one recent series was 53 months (Wagner et al, 2008). The risk of developing a second primary tumor of the retained urethra following cystectomy is influenced by the presence and extent of involvement of the prostatic urethra and prostatic stroma, with stromal invasion carrying the highest risk of approximately 30% (none < focal CIS < diffuse CIS < ductal/acinar involvement < stromal invasion) (Hardeman and Soloway, 1990). Urethrectomy should be considered in men if CIS is diffuse within the prostatic urethra or ducts or if there is invasion of prostatic stroma. One method for assessing the risk to the retained urethra and determining the appropriate choice of urinary diversion is to perform transurethral resection biopsies of the prostatic urethra (see “Staging the Bladder and Urethra” earlier) (Wood et al, 1989; Lerner and Shen, 2008b). Some experts, however, believe that the only absolute indication for consideration of urethrectomy is cancer at the apical urethral margin (Donat et al, 2001; Stein et al, 2005). Small low-grade papillary tumors of the urethra can be resected before cystectomy and the urethra retained if there is no other indication for urethrectomy. The probability of developing a second primary TCC of the retained urethra is lower with orthotopic diversion than with cutaneous diversion (Stein et al, 2005). Cystectomy in women has historically included the urethra. New data indicate that the distal two thirds of the urethra serves as an adequate sphincter mechanism innervated by the pudendal nerve and is infrequently involved with transitional cell cancer (Stenzl et al, 1995; Stein et al, 1998). Limiting dissection only to the soft tissue above the endopelvic fascia will preserve the innervation crucial to maintaining the sphincter function of the urethra. Cancer at the bladder neck or urethra or a T4 tumor involving the anterior vagina is a contraindication to urethral preservation and creation of an orthotopic neobladder. Posterior T3 tumors are a relative contraindication, particularly if the tumor involves the trigone, because achieving an adequate margin is difficult. Urethral cancer is always associated with bladder neck cancer, so findings of a biopsy of the bladder neck are an excellent surrogate for preoperative staging of the female urethra (Fig. 82–3). The proximal one third of the urethra is resected en bloc with the cystectomy specimen in order to reduce the risk of urinary retention, leaving the distal two thirds to provide adequate sphincter function. Pathologic tumor stage and nodal status are the primary variables affecting the risk of progression and survival probability following cystectomy (Table 82–3) (Stein et al, 2001; Madersbacher et al, 2003; Hautmann et al, 2006; Shariat et al, 2006b; Ghoneim et al, 2008; Manoharan et al, 2009). The incidence of nodal metastasis increases with depth of invasion, and lymphovascular invasion increases the risk of nodal metastasis and is an independent predictor of outcome (Quek et al, 2005; Algaba, 2006; Herrmann et al, 2008). Positive surgical margins have an adverse impact on outcome as well (Dotan et al, 2007; Sonpavde et al, 2009). Recent studies suggest that improved outcomes are associated with high-volume surgeons and hospitals and that patient’s age and body mass index (BMI) may also affect morbidity and survival (Konety et al, 2005; Nielsen et al, 2007; Bagrodia et al, 2009). Table 82–3 Percentage 5-Year Disease-Specific Survival (DSS) by Pathologic Stage after Radical Cystectomy with and without Pelvic Lymph Node Metastasis: Selected Series Reporting DSS (2000-2009) The majority of randomized clinical trials evaluating perioperative chemotherapy has been underpowered, used ineffective chemotherapy, or have had methodological flaws (Sylvester and Sternberg, 2000). However, more sufficiently powered neoadjuvant studies and meta-analyses provide further recommendations (Hall, 2002; Grossman et al, 2003b; Advanced Bladder Cancer Meta-analysis Collaboration, 2005b). Chemotherapy before surgery has several advantages. Therapy is better tolerated before surgery or radiation. Chemotherapy-related toxicities are considerably less in patients with localized disease than in those with metastatic disease on the basis of performance status. Patients are often able to tolerate a greater dose intensity and more cycles of chemotherapy preoperatively than postoperatively. Neoadjuvant chemotherapy allows in vivo drug sensitivity testing that may provide useful information for later therapy. The primary tumor can be evaluated for response, which also has major prognostic significance. In addition, preoperative chemotherapy may down-stage tumors, potentially allowing for technically easier surgery (Calabrò and Sternberg, 2009b). Evaluating response to neoadjuvant chemotherapy can be complicated by discrepancies between clinical and pathologic staging, which have been reported in some 30% of cases (Herr and Scher, 1990; Sternberg et al, 2003). The major disadvantage of neoadjuvant chemotherapy is a delay in definitive local therapy in patients who do not respond or whose disease progresses. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy has even been associated with a poorer outcome (Sanchez-Ortiz et al, 2003; Stein, 2003a). Another theoretic disadvantage of neoadjuvant chemotherapy is the possible increase in the incidence of perioperative morbidity. Few reports have studied this (Hall et al, 1996; Millikan et al, 2001). In a comparative study of neoadjuvant and adjuvant chemotherapy, neoadjuvant chemotherapy did not increase perioperative morbidity (Millikan et al, 2001). In the United States and in most of Europe, radical cystectomy is the preferred treatment for fit patients who have a good performance status. Many randomized trials have evaluated the impact of neoadjuvant chemotherapy on survival. These trials have shown either a trend toward a benefit or no survival advantage. The majority of these trials have, however, been underpowered. Randomized neoadjuvant chemotherapy trials are summarized in Table 82–4 (Sternberg et al, 2007). When results were first published, there was a nonsignificant trend toward improvement in survival in patients treated with neoadjuvant CMV chemotherapy (International Collaboration of Trialists, 1999). In an update presented at the American Society of Clinical Oncology (ASCO) meeting in 2002, with longer follow-up of 7.4 years, the data have barely reached statistical significance (P = .048). There was a 5.5% benefit in favor of patients treated with CMV (Hall, 2002). Survival at 5 years was 50% compared with 44%, and at 8 years was 43% versus 37%. Following neoadjuvant chemotherapy, no residual tumor or pathologic complete response (pCR) was found in 32.5% of cystectomy specimens. Chemotherapy-related mortality was 1%. Chemotherapy did not increase the rate of postoperative complications. Although patients treated with CMV had a survival benefit that was maintained over time, it was concluded that there was no change in absolute benefit. In the Southwest Oncology Group (SWOG) Intergroup trial, 317 patients with clinical T2 to T4a bladder cancer were randomized to receive cystectomy or three cycles of neoadjuvant M-VAC chemotherapy followed by cystectomy (Grossman et al, 2003a). Enrollment took place at 126 institutions over 11 years. Patients were stratified according to age (younger than 65 years old or 65 years and older) and stage (cT2 vs. cT3 or cT4a). The aim of the study was to detect an increase in survival with M-VAC chemotherapy. The original study was planned for one-sided testing, which means that the investigators were planning to test not for a difference but only for improvement because the hypothesis was that medical practice would change only if there was an improvement with chemotherapy. Median survival among patients randomized to surgery alone was 46 months, as compared with 77 months for patients who received neoadjuvant M-VAC chemotherapy (P = .06; two-sided stratified log rank test). In part because of the very long accrual period and follow-up, these results have achieved borderline statistical significance. The estimated risk of death was decreased by 25% (HR = 1.33) in patients treated with neoadjuvant M-VAC (Grossman et al, 2003b). The neoadjuvant chemotherapy group had a significantly higher proportion of patients with pCR than the cystectomy-alone group (38% vs. 15%, P < .001). Patients with locally advanced (cT3 or T4a) disease had the greatest survival benefit from neoadjuvant chemotherapy (65 vs. 24 months). Other randomized trials of neoadjuvant chemotherapy are found in Table 82–4 (Malmstrom et al, 1996; Bassi et al, 1998; Sherif et al, 2002). The majority of neoadjuvant randomized trials have failed to show an unequivocal survival benefit in favor of chemotherapy. However, the majority of the studies have been hampered by inadequate sample sizes, suboptimal chemotherapy, premature closure, or inadequate follow-up (Sylvester and Sternberg, 2000). For this reason, meta-analyses have attempted to explain and interpret these data (International Collaboration of Trialists, 1999; Winquist et al, 2004). The Advanced Bladder Cancer (ABC) Meta-analysis included data on 3005 patients from 11 randomized neoadjuvant chemotherapy trials (Advanced Bladder Cancer Meta-analysis Collaboration, 2005b).The overall analysis was in favor of chemotherapy (HR = .89, 95% CI .81 to .98, P = .022). In patients who received single-agent cisplatin chemotherapy, neoadjuvant chemotherapy appeared detrimental, but the numbers were too small to support definitive conclusions (P = .264). However, in the subset of patients who received cisplatin-based combination neoadjuvant chemotherapy, the meta-analysis showed a reduction in the risk of death of 14% (HR = .86, 95% CI .77 to .95, P = .003), translating into a 5% absolute survival benefit at 5 years (95% CI: +2% to +9%). The results are similar to the results obtained in the EORTC and MRC trial, which was the largest trial in the analysis (Hall, 2002). The authors noted no difference in the relative risk reduction either in relation to the type of local treatment (cystectomy, radiotherapy or the combination) or in relation to patient characteristics. However, apart from this study, neoadjuvant chemotherapy before radiation therapy has not been shown to improve survival (Zietman et al, 1997). Despite the 5% survival benefit, the neoadjuvant approach before cystectomy has not been widely accepted. In an analysis of stage III bladder cancer from the National Bladder Cancer Database (n = 11,328), only 0.7% of patients received neoadjuvant chemotherapy (David et al, 2007). Part of the reason may be that physicians feel that it is not worthwhile to give chemotherapy to all patients to achieve a 5% benefit (Sternberg and Collette, 2006a). The studies on which recommendations have been made do not contain information on toxicity and quality of life. In most of the trials elderly patients (median age 63 to 65 years in EORTC, SWOG, Nordic) or those with poor renal function or performance status have not been included, making it difficult to generalize the results to all of the elderly patients who are affected with bladder cancer (Droz, 2005). Information is lacking on subgroups of patients to indicate who would derive the most benefit from treatment. In some studies (Nordic) both neoadjuvant and adjuvant chemotherapy were used. And of perhaps most importance, the impact of cystectomy and the quality of surgery have been shown to be as important as whether or not chemotherapy was administered (Herr, 2004). The delay in definitive surgery in patients who do not respond to treatment raises concerns regarding compromise of curability, and many urologists simply believe that a potential 5% advantage in overall survival is not great enough to justify giving toxic chemotherapy to all patients before surgery. Many oncologists prefer to use newer agents such as gemcitabine or the taxanes, which have not been evaluated in randomized studies. A single-institution retrospective report of neoadjuvant gemcitabine and cisplatin before cystectomy reported that 26% of patients achieved a pathologic complete response to chemotherapy, which may be comparable with other combination cisplatin-based regimens (Dash et al, 2008). In patients with pT3-4 and/or N+M0 disease, 5-year survival after radical cystectomy is only 25% to 35% at best. As a result, adjuvant chemotherapy has been advocated for high-risk patients in an effort to delay recurrence and prolong survival. Delivery of chemotherapy postoperatively has potential advantages. An adjuvant approach allows selection of patients at highest risk of metastatic or recurrent disease on the basis of an accurate pathologic evaluation (Calabrò and Sternberg, 2009b). Surgery is performed without delay, and the advent of orthotopic neobladders and continent urinary diversions has improved quality of life in patients after cystectomy, favoring immediate cystectomy. There is evidence that delaying cystectomy can be detrimental (Chang et al, 2003; Sanchez-Ortiz et al, 2003), and no time is wasted in those patients who do not respond to chemotherapy. The availability of sufficient tissue for increasingly sophisticated analysis of molecular prognostic and predictive markers is also a potential advantage. If micrometastases are present, they can be treated with chemotherapy when at a low volume, rather than after there is overt metastatic disease. Several factors contribute to the difficulty in accrual to bladder cancer trials. Patients are often elderly with significant comorbidities that are related to smoking and/or compromised renal function (Jin et al, 2006). An additional disadvantage is the difficulty in administering chemotherapy to those with surgical morbidities following cystectomy. A prospective standardized reporting methodology was used to quantify and characterize the risk of perioperative morbidity following cystectomy at MSKCC, a center with high-volume, fellowship-trained urologists. Staggering rates of postoperative complications were observed within 90 days of surgery (Donat et al, 2009; Shabsigh et al, 2009), further confirming prior observations at another high-volume center in California (Stein and Skinner, 2006). These often underreported postoperative morbidities can lead to long delays and difficulties in administering adjuvant chemotherapy. Despite its appeal, there have been few randomized trials evaluating adjuvant chemotherapy. Table 82–5 shows the results of randomized trials of adjuvant chemotherapy after cystectomy (Logothetis et al, 1988; Skinner et al, 1991; Stockle et al, 1992; Studer et al, 1994; Stockle et al, 1995; Freiha F et al, 1996; Bono et al, 1997; Otto et al, 2001; Cognetti et al, 2008). All of these trials were relatively small, enrolling only 49 to 108 patients. Nonetheless, two trials suggest a survival benefit with adjuvant chemotherapy.
Clinical Presentation, Diagnosis, And Evaluation
Natural History
Histology
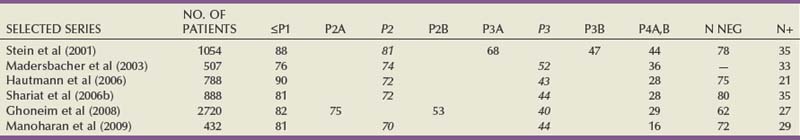
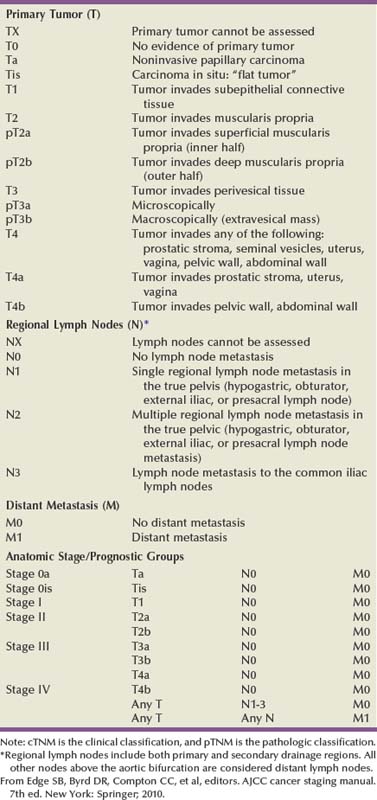
Clinical and Pathologic Staging
Staging Pitfalls
T1 versus T2
T2 versus T3
Staging Tumors of the Bladder and Urethra
Staging Evaluation: Regional and Metastatic Disease

Treatment: Surgical
Radical Cystectomy and Bilateral Pelvic and Iliac Lymphadenectomy
Extent of Pelvic and Iliac Node Dissection
What Constitutes an Adequate Lymph Node Dissection?
Staging
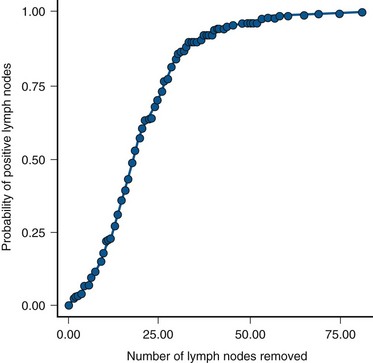
Is There a Threshold of Number of Nodes Removed in Order to Identify the Majority of N+ Patients?
Outcome Related to Nodal Status
Intraoperative Decision Making
Carcinoma in Situ of the Ureter
Prostatic Transitional Cell Carcinoma and Managing the Apical Urethral Margin
Managing the Female Urethra
Survival and Treated Natural History
Neoadjuvant Chemotherapy
Randomized Trials of Neoadjuvant Chemotherapy
Meta-Analyses of Randomized Neoadjuvant Chemotherapy Trials
Adjuvant Chemotherapy
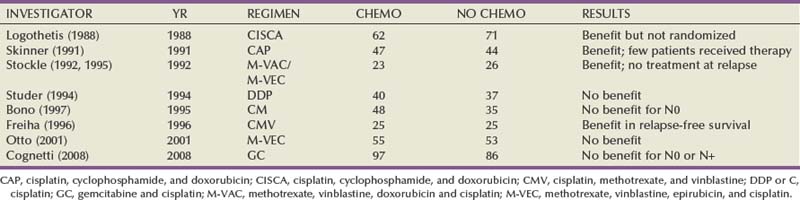
Randomized Trials of Adjuvant Chemotherapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Management of Metastatic and Invasive Bladder Cancer

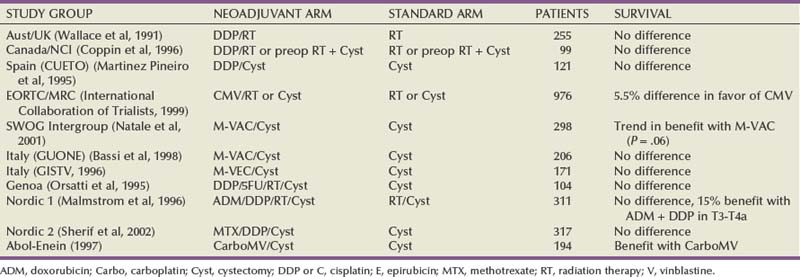
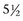 years from 106 institutions were randomized to receive cisplatin, methotrexate, and vinblastine (CMV) neoadjuvant chemotherapy or no chemotherapy. Cystectomy and/or radiation therapy was permitted as definitive management of the primary tumor. The study was designed to detect an absolute improvement in survival of 10% (from 50% to 60%) with a power of 90% and a type 1 error of 5%.
years from 106 institutions were randomized to receive cisplatin, methotrexate, and vinblastine (CMV) neoadjuvant chemotherapy or no chemotherapy. Cystectomy and/or radiation therapy was permitted as definitive management of the primary tumor. The study was designed to detect an absolute improvement in survival of 10% (from 50% to 60%) with a power of 90% and a type 1 error of 5%.