Edmund Sabanegh, Jr., MD, Ashok Agarwal, PhD Over the past 50 years, we have witnessed dramatic advances in the understanding and treatment of male fertility. The introduction of intracytoplasmic sperm injection (ICSI) in 1992 (Palermo et al, 1992), a technique of in-vitro fertilization using direct insertion of a single sperm into an egg, offered the ability to bypass even some of the most severe etiologies of male subfertility but raised a variety of cost and safety issues. Growing understanding of the genetics of fertility, environmental influences on gonadocytes, and the endocrine basis for germ cell development holds promise to allow more targeted diagnostic and therapeutic interventions. Unlike many other disease states, fertility represents a complex interaction between two individuals involving multiple organ systems. Attempts to isolate pathology to one gender are confounded by the fact that male fertility is not a clearly quantifiable parameter but is dependent on requirements of the individual female reproductive system. Older studies have related 20% of cases of infertility to purely male factor etiology, while an additional 30% to 40% involve both male and female factor pathology (Simmons, 1956). Newer studies have shown little change in this distribution with more than 50% attributable to male factor, despite advances in the diagnosis and management of infertility (Mosher and Pratt, 1991; Thonneau et al, 1991). Knowledge of the inherent inefficiency of normal human reproduction is critical before we can define infertility. Studies of conception in normal couples reveal that 60% to 75% will conceive within 6 months of unprotected intercourse and 90% by 1 year (Tietze et al, 1950; Spira, 1986). On the basis of this, the classic definition of infertility became the absence of conception after 12 months of regular, unprotected intercourse, a definition supported by the Practice Committee of the American Society for Reproductive Medicine (ASRM). Because a small number of normal couples will conceive between 1 and 2 years, the World Health Organization (WHO) recommends 24 months of unprotected intercourse as the preferred definition of infertility (Rowe, 1993). Geographically diverse population-based studies have reported a remarkably consistent 15% to 20% incidence of infertility (WHO, 1991; Gunnell and Ewings, 1994; Philippov et al, 1998). Although the classic definition of infertility would suggest deferring medical assessment until 12 months of unprotected intercourse, we support performance of a basic, cost-effective evaluation of both partners at the time of presentation for evaluation. Current recommendations by the Practice Committees of the American Urological Association and the American Society for Reproductive Medicine (Male Infertility Best Practice Committee Report, 2006a, 2006b) recommend infertility evaluation before 1 year if (1) male infertility risk factors such as a history of bilateral cryptorchidism are known to be present, (2) female infertility risk factors including advanced female age (older than 35 years) are suspected, or (3) the couple questions the male partner’s fertility potential. A timely but limited evaluation provides early identification and correction of factors that may reduce fertility, as well as reassurance in an emotionally difficult situation for couples. Alleviation of anxiety related to infertility may in itself provide therapeutic value. Whenever possible, treatment should involve correction of a specific problem rather than blanket application of costly assisted reproductive technologies. ART use for male factor infertility in the United States has been estimated to cost almost $18 billion dollars in 1 year alone (Meacham et al, 2007), underscoring the need for addressing the specific cause of male factor infertility. More importantly, ART application without attention to the male factor may mask potentially significant and even life-threatening conditions present in the infertile male, conditions that may occur in up to 1.3% of men and that would only have been diagnosed with a complete medical evaluation (Kolettis and Sabenegh, 2001). However, ART retains an important role in the management of male factor infertility, especially where no etiology for infertility can be identified or in the setting of noncorrectable causes. In addition to assisted reproduction, donor sperm insemination and adoption remain excellent options for the management of noncorrectible infertility. Successful diagnosis and treatment of infertility requires careful attention to obtaining a thorough history. A diverse variety of specific factors can affect subsequent fertility or sexual function (Table 21–1). Although focus may be on long-term factors that can affect fertility, human spermatogenesis is estimated to involve a 64-day cycle with an additional 5 to 10 days of epididymal transit time on the basis of radioisotope labeling studies (Clermont and Heller, 1963; Franca et al, 2005; Misell et al, 2006). Factors such as fever, illness, or drug use in the several months before semen testing should prompt repeat testing after an additional 3 months to rule out transient detrimental effects. Table 21–1 Pertinent History in Evaluation of the Infertile Male The timing and frequency of intercourse are an important component of the reproductive history. In recent years, the ease and availability of ovulation predictor kits, which measure midcycle urinary luteinizing hormone (LH) surge as a predictor of impending ovulation, have allowed couples to approach reproductive timing in a more informed and effective fashion. However, many couples are not aware of the viability of spermatozoa within the female reproductive tract with sperm surviving between 2 and 5 days in favorable cervical mucus (Wilcox, 1995). This finding is the basis for the widely offered recommendation of intercourse frequency every 2 days near the time of ovulation, maximizing the chance that viable sperm are available to the oocyte (Tur-Kaspa et al, 1994). Intercourse that is too frequent does not allow replenishment of adequate numbers of spermatozoa within the epididymis, whereas infrequent intercourse may miss the potential window for fertilization. An assessment of erectile and ejaculatory function is also germane to the initial evaluation. Use of vaginal lubricants is commonplace in reproductive-aged couples with almost one half of couples reporting intermittent use (Oberg et al, 2004). A number of commercially available lubricants that are not marketed as spermicidal agents have been shown to adversely affect sperm motility (Miller, 1994; Kutteh et al, 1996; Anderson et al, 1998) and sperm deoxyribonucleic acid (DNA) integrity (Agarwal et al, 2008). Although some lubricants such as vegetable oil, raw egg white, and Pre-Seed have minimal spermicidal effect (Goldenberg and White, 1975; Edvinsson et al, 1983; Agarwal et al, 2008), it remains optimal to avoid lubricant use if possible and to use minimal concentrations of the least toxic lubricant available, if required. A variety of pediatric conditions including cryptorchidism, postpubertal mumps orchitis, and testicular torsion or trauma can have significant implications on eventual fertility. Although prepubertal mumps is unlikely to have detrimental effects on fertility, mumps occurring in the postpubertal timeframe is associated with unilateral or bilateral orchitis in up to 40% of children (Werner, 1950) with potentially devastating testicular damage. Testicular torsion or trauma can result in testicular atrophy, as well as the development of antisperm antibodies, which are detrimental to sperm function and motility (Bronson et al, 1984; Puri et al, 1985). Prior scrotal, inguinal, or retroperitoneal surgeries can obstruct the ductal system or interfere with emission or ejaculation of sperm. Classic retroperitoneal lymphadenectomy for testicular cancer frequently results in sympathetic nerve injury leading to anejaculation or retrograde ejaculation (Kedia et al, 1977). Fortunately, with modifications in the surgical template and intentional nerve sparing, ejaculation can be preserved in almost all patients with low-stage disease and in selected patients with more advanced disease (Donohue et al, 1990). Bladder neck surgery and transurethral resection of the prostate can lead to retrograde ejaculation due to bladder neck incompetence. In selected patients, transurethral incision of the prostate can allow preservation of antegrade ejaculation. Vasal injuries from inguinal surgery have seen resurgence with the popularity of polypropylene mesh hernia repairs, which can induce dense fibroblastic reactions leading to vasal obstruction (Shin, 2005). Systemic diseases in adulthood can affect fertility through a number of different mechanisms. Diabetes mellitus, spinal cord injuries, and multiple sclerosis exert effects through impairment of both ejaculatory and erectile function (Sønksen and Biering-Sørenson, 1992; Sexton and Jarow, 1997). Diseases of the thyroid, both hyper and hypo function, affect both steroid hormone metabolism and sperm quality and have been associated with subfertility (Velazquez and Bellabarba, 1997; Abalovich et al, 1999; Krassas et al, 2002). Subclinical hypothyroidism does not produce significant seminal abnormalities (Trummer et al, 2001). Neoplasms in general can induce marked impairment of spermatogenesis due to endocrine disturbances, malnutrition, hypermetabolism with associated fever, and immunologic factors (Costabile and Spevak, 1998; Wong et al, 2000). In addition to the global effects of malignancy on reproductive health, specific malignancies such as Hodgkin disease (HD) and testicular germ cell tumors produce significant direct gonadotoxic effects (Petersen et al, 1999; Rueffer et al, 2001). Pretreatment testicular dysfunction associated with HD has been postulated to be due to a variety of mechanisms including genetic abnormalities at the germ cell level, endocrinopathies, systemic release of cytokines injurious to both the seminiferous tubules and the Leydig cells, and negative local effects from intratesticular lymphatic tissue. Testicular tumors impair spermatogenesis by the destruction of surrounding tissue, local secretion of HCG and other paracrine factors, intrascrotal temperature elevation, and alterations in the local blood flow. Cancer treatments including chemotherapy and radiation produce direct toxicity on surviving germ cells, potentially depressing spermatogenic function for many years if recovery occurs at all (Nalesnik et al, 2004; Ståhl et al, 2006). A detailed history should include a comprehensive assessment of medications, recreational, environmental, and occupational exposures that can impact fertility. Medications can impair fertility by direct toxic effects on gonadocytes, disturbance of the hypothalamic-pituitary-gonadal axis, disruption of ejaculatory or erectile function, and inhibition of libido. Antibiotics including nitrofurantoin, erythromycin, tetracycline, and gentamycin exhibit direct gonadotoxicity or impair sperm function. Androgen production is inhibited by spironolactone, ketoconazole, and cimetidine (Griffin and Wilson, 1991). Treatments for ulcerative colitis such as sulfazalazine are associated with reversible reductions in sperm concentration and motility (Toth, 1979). α Blockers, which are commonly used for treatment of benign prostatic hypertrophy and hypertension, are associated with retrograde ejaculation, an effect that may be more prominent with tamsulosin than with other selective α blockers (Giuliano, 2006). 5-α reductase inhibitors such as finasteride and dutasteride inhibit conversion of testosterone to the metabolically active dihydrotestosterone and are commonly used for treatment of benign prostatic hypertrophy. Use of these agents has been associated with reductions in semen volume, as well as erectile and ejaculatory dysfunction (Giuliano, 2006). Psychotherapeutic medications including the selective serotonin reuptake inhibitors (SSRI), monoamine oxidase inhibitors, phenothiazines, and lithium can suppress the hypothalamic-pituitary-gonadal axis, impair ejaculation and erectile function, and reduce libido (Nudell et al, 2002). Exogenous testosterone and steroid supplementation, whether medically prescribed or used for recreational purposes, can have the most profound detrimental effects on spermatogenesis of the medical agents. Androgenic agents induce hypogonadotropic hypogonadism leading to azoospermia, which can last 6 months or more after cessation of the supplements and, on occasion, may be irreversible (Sigman et al, 2006). Testosterone replacement therapy in hypogonadal men desiring fertility should be avoided, and alternate regimens such as antiestrogens (clomiphene citrate, tamoxiphene) should be considered instead. Recreational drugs have also been implicated as gonadotoxic agents. Marijuana use is associated with gynecomastia, reductions in serum testosterone, decreased sperm counts, and elevated seminal leukocytes (Harmon and Aliapoulios, 1972; Hembree et al, 1979; Close et al, 1990). Abnormal sperm morphology, decreased motility, and low sperm concentrations have been associated with cocaine use (Bracken et al, 1990; Hurd et al, 1992). Although long-term abuse of alcohol is associated with global suppression of the hypothalamic-pituitary gonadal axis and spermatogenesis, moderate intake is not associated with significant deterioration in fertility (Muthusami and Chinnaswammy, 2005). Although the role of smoking in lung and heart diseases is widely established, the adverse effect of tobacco on male reproductive health is less well known by the general public. Smoking is associated with declines in basic semen parameters such as sperm concentration, viability, forward motility, and morphology (Vine et al, 1996; Künzle et al, 2003), as well as declines in sperm penetration ability and hence fertilization rates (Sofikitis et al, 1995). Defects in these parameters not only affect normal fecundity but also lower assisted reproduction success rates (Joesbury et al, 1998; Zitzmann et al, 2003). The impacts of environmental and occupational exposures on spermatogenesis are more difficult to prove and quantify. Certain agents such as heavy metals, pesticides such as dibromochloropropane, organic solvents, and heat have been widely associated with gonadotoxicity (Lipshultz and Corriere, 1980; Moreira and Lipshultz, 2008). Industrial lead exposure exerts direct negative effects on both seminiferous tubules and the hypothalamic pituitary axis, resulting in asthenospermia, oligospermia, teratospermia, and ultimately reduced fertility (McGregor and Mason, 1990; Gennart et al, 1992; Shiau et al, 2004). Inflammatory diseases can have profound effects on the patency of the genital tract and function of the spermatozoa. Infectious diseases such as prostatitis or sexually transmitted infections such as Chlamydia or Neisseria gonorrhea are associated with elevated seminal oxidative stress and leukocytospermia, resulting in abnormal bulk semen parameters, elevated sperm DNA fragmentation, and reduced fertility (Trum, 1998; Pasqualotto, 2000; Aitken et al, 2007). A history of bilateral epididymitis with subsequent azoospermia suggests the possibility of epididymal obstruction. Epididymal granuloma may result from noninfectious diseases such as sarcoidosis (Rao, 2009) or from the sequelae of an active tuberculosis infection. Epididymal sarcoidosis has been associated with azoospermia, which may be reversible with corticosteroid treatments (Svetec, 1998). Questions regarding a family history of infertility are an underemphasized component of the initial assessment because of a misperception that genetic conditions that cause infertility are inherently nontransmissible. A family history of cystic fibrosis may suggest the diagnosis of congenital bilateral absence of the vas deferens (CBAVD) with its associated vasal, epididymal, and seminal vesicle anomalies. Abnormalities of the androgen receptors should be considered in the setting of a family history of intersex disorders. Today’s widespread use of assisted reproductive technologies such as ICSI allow us to overcome subtle genetic abnormalities that may account for many cases of idiopathic male subfertility. With up to 2% to 4% of European and more than 1% of U.S. children born today (Wright et al, 2007) as a result of these technologies, we would expect the genetic causes of infertility to represent a growing etiology of infertility as these men attempt to conceive in the future, further reinforcing the importance of obtaining a comprehensive family history. Finally, a complete history should also include an assessment of female factor fertility issues because almost two thirds of infertility can be attributed to the female side, either wholly or in combination with male factors. Failure to incorporate these considerations into the evaluation and management can result in ineffective and unnecessarily expensive treatment courses. Risk factors for female subfertility include but are not limited to advanced age, irregular menstrual cycles, and a history of pelvic pathology including endometriosis and pelvic infections. Fecundity begins to decline sharply after age 35 and is less than 5% by age 40 (Robins and Carson, 2008). Ovulatory dysfunction occurs in 40% of infertile women, accounting for the largest single cause of female infertility (Mosher and Pratt, 1991). A variety of tools are used to assess ovulation; these include basal body temperature charts, midluteal serum progresterone levels, endometrial biopsy, urinary LH prediction kits and transvaginal sonographic detection of ovarian follicles. Tests of ovarian reserve involve an assessment of remaining ovulatory capability and a de facto assessment of ovarian aging. Standard testing includes basal hormone measurements of follicle-stimulating hormone (FSH) and estradiol, as well as dynamic ovarian testing, which involves stimulation of ovulation using clomiphene citrate or gonadotropin (Hofmann et al, 1996). Abnormalities of uterine cavity or tubal anatomy occur in up to 25% of infertile women (Thonneau et al, 1991). Uterine and tubal patency can be assessed with hysterosalpingography (HSG) or laparoscopy with chromotubation. HSG testing involves transcervical injection of contrast material allowing assessment of intrauterine and tubal anatomy. In addition, a number of reports have suggested that the use of oil-based contrast materials may be therapeutic, in addition to the diagnostic value (Al-Fadhli et al, 2006; Luttjeboer et al, 2007). Laparoscopy allows confirmation of HSG findings by direct observation of free spill of contrast (methylene blue or indigo carmine introduced via cervix) and detection of other pathologies such as endometriosis, fibrial phimosis, or peritubular adhesions. At the time of laparoscopy, tubal reconstruction and surgical ablation of endometriosis may be undertaken. Genital examination starts with a careful examination of the phallus. Penile curvature, chordee, or hypospadias may interfere with semen deposition in the vaginal vault. A careful examination of the scrotal contents is the most critical part of the examination. The testes should be examined with the patient in both supine and standing positions in a warm room to assist relaxation of the cremasteric muscle. The entire testicular surface should be carefully palpated to assess consistency and rule out masses because infertility has been consistently established as a risk factor for testicular carcinoma (Kolettis and Sabanegh, 2001). Testicular size should be assessed with either an orchidometer, calipers, or sonographic measurement. Normal adult testicular measurements have been established to be at least 4 × 3 cm or 20 mL in volume (Charny, 1960). Because 85% of the testicular volume involves sperm production, decreased testicular size portends impaired spermatogenic potential (Lipshultz and Corriere, 1977). The epididymides should be carefully palpated for enlargement or induration, which can indicate downstream obstruction or inflammatory conditions such as epididymitis. Granulomatous changes of the epididymis have been associated with tuberculosis, bacile Calmette-Guerin (BCG) treatments, and sarcoidosis. Small cystic lesions of the epididymis are common and are usually spermatoceles, which are often nonobstructing. Papillary cystadenomas are less commonly seen and may present in conjunction with von Hippel-Lindau (VHL) disease. Examination of the spermatic cord in the supine and standing position allows the detection of varicoceles, defined as abnormally dilated scrotal veins. Varicoceles are detected by palpation for assymetry of the spermatic cord, or an impulse, during the Valsalva maneuver. Gentle traction on the testis during this examination can be helpful in more difficult examinations such as patients with high riding testes or exaggerated cremasteric muscle response to Valsalva. Varicoceles are present in 15% of normal males, 19% to 41% in men presenting with primary infertility, and up to 81% of men with secondary infertility (Agarwal et al, 2007). Varicoceles are graded by size with small grade I varicoceles, which are detectable only during the Valsalva maneuver; moderate size grade II varicoceles, which can be palpated without Valsalva; and the large grade III varicoceles, which are visible through the scrotal skin and classically described as feeling like a “bag of worms.” Due to the right-angle insertion of the left gonadal vein into the renal vein with the resulting turbulent flow, varicoceles are more prevalent on the left side with almost 90% presenting on the left side alone. Large unilateral right-side varicoceles and varicoceles that fail to decompress with the supine position suggest the possibility of retroperitoneal or caval pathology such as renal neoplasms and warrant dedicated imaging. A variety of ancillary procedures including ultrasonography with and without Doppler examination, radionucleotide scans such as technetium 99m pyrophosphate, thermography, and venography have been used to corroborate clinical examination findings. In the absence of physical examination findings, varicoceles detected by these procedures alone are considered subclinical and not of clinical significance. Physicians should provide patients with standard guidelines for the collection of semen because suboptimal sperm collection remains a frequent cause of error in semen analysis. There should be 2 to 7 days of sexual abstinence before collection. Two separate samples at least 7 days apart should be analyzed (Rowe, 2000; Jeyendran, 2003). The duration of abstinence should be constant, if possible, because each additional day can add as much as 25% in sperm concentration (Carlsen et al, 2004). Lubricants should be avoided because they may interfere with motility results. Coitus interruptus should be discouraged because it often leads to inaccurate results (i.e., the first part of the ejaculate, which contains most of the sperm, may be lost). Masturbation in a clinical setting is the recommended procedure. Collection is done in a private room in the same facility where the semen will be analyzed. The glans and the penis should be cleaned with a wet paper towel (soap should be avoided). Lubricant use is discouraged but, if necessary, should not be applied to the glans. A clean, sterile container should be used for specimen collection. The container should be provided by the laboratory to avoid contamination or spermicidal effects. The main advantages of this collection method are its simplicity, noninvasiveness, and inexpensiveness (Jeyendran, 2003). Some men may not be able to achieve adequate erection and ejaculation. Assistance can be provided to them by oral medications such as phosphodiesterase type 5 inhibitors given 30 to 60 minutes before collection. Cavernosal and subcutaneous injections of prostaglandins are less popular but remain possible options for patients who have erectile dysfunction. Seminal pouches that do not contain any spermicides allow the patient to engage in sexual activity if he is incapable of or uncomfortable producing specimens by masturbation. Vacuum erection devices can also be used to obtain erection by creating a vacuum around the penis, generating a pressure differential that fills the corpora with blood. Vibratory stimulation may be used for patients who have suffered spinal cord injury, if the spinal cord lesion is T8 and above (Brown et al, 2006). Rectal probe electro-stimulation induces ejaculation by stimulation of the efferent fibers of the hypogastric plexus. Precautions for autonomic dysreflexia should be taken while doing these procedures because some patients with high spinal cord lesions (T6 and above) can have life-threatening hypertension (Jeyendran, 2003). The semen analysis characteristics can be classified into two groups: macroscopic and microscopic. The five macroscopic measurements in a standard sperm analysis have remained fairly constant, with the normal values remaining relatively unchanged since the inception of the semen analysis in the 1950s (Table 21–2). Normal human semen is an off-white to grayish-yellow opalescent fluid. In event of urine contamination, the semen sample has a yellow discoloration. The semen may appear pink in patients with urethral bleeding and yellowish in jaundice patients. During the time of ejaculation, the spermatozoa are suspended in the secretions of prostate, seminal vesicles, bulbo-urethral glands, and other accessory glands that form a coagulum. The specimen usually liquefies within 30 minutes. However, semen obtained from patients with congenital bilateral absence of the vas usually does not form a coagulum and is acidic. Liquefaction is aided by the proteolytic enzyme fibrinolysin, secreted by the prostate. Improper or prolonged liquefaction indicates an ejaculatory duct obstruction or poor prostatic secretion. Viscosity and nonliquefaction are two different phenomena often confused. Viscosity relates to the fluid nature of the sample. It is measured by dropping the semen sample into a container using a pipette and observing the length of the thread formed. Increased viscosity is often associated with infertility because it is known to impair sperm movement. Semen samples that are highly viscous can be treated with enzymes such as trypsin before they are processed for therapeutic purposes. Measurement of pH is a standard component of semen analysis and is largely determined by the secretions from the seminal vesicles and the prostate. The normal range of pH has been defined as 7.2 to 8.0. Because the secretions of seminal vesicles are alkaline, acidic pH indicates congenital absence of the vas with the associated seminal vesicle hypoplasia seen in azoospermic patients (WHO, 1999). The microscopic examination starts with the creation of a wet smear by placing a drop of semen on a slide covered with a cover slip and observing it under 1000× magnification. Sperm agglutination, sperm presence, and subjective motility can be assessed by this method. Sperm adhesion to nonsperm elements (nonspecific agglutination) may indicate accessory gland infection. Sperm-to-sperm agglutination (site-specific agglutination) can be secondary to antisperm antibodies; however, it should be kept in mind that a small degree of agglutination is normal (WHO, 1999). When agglutination is observed, semen cultures and antibody assessment should be performed. When oligospermia is reported, the levels of motility and morphology become especially important. Total motile sperm counts guide decisions on appropriate therapies including the use of ARTs. In cases of azoospermia and severe oligospermia, hormonal evaluation (FSH and testosterone) should be requested. Karyotyping and Y microdeletion may provide valuable information regarding the etiology of the patient’s abnormal semen parameters and important information if in-vitro fertilization (IVF) is being entertained as a treatment option. Foci of microdeletions in the Y chromosome are associated with impaired spermatogenesis and, depending on their location, may predict poor sperm retrieval even with testicular biopsy. Karyotyping may also detect autosomal or X-linked genetic aberrations causing infertility. Knowledge of the chromosome status is important because male offspring conceived with intracytoplasmic sperm insemination (ICSI) or even natural conception most likely will inherit the same microdeletion (Krausz et al, 2000). Motility is recognized as the most important predictor of the functional aspect of spermatozoa. Sperm motility is a reflection of the normal development of the axoneme and the maturation that it undergoes within the epididymis. This parameter is subject to significant potential for technical mistakes in the laboratory. The method most commonly employed by laboratories is the simple estimation of the motility of sperm on several fields. This subjective assessment is prone to inaccuracy. Moreover, in-vitro motility of sperm may not reflect the true motility within the female reproductive tract. The sperm motility is graded according to the WHO as follows: A—Rapid forward progress motility; B—Slow or sluggish progressive motility; C—Nonprogressive motility; and D—Immotility. The cutoff value for normal is 50% grade A+B or 25% grade A motility (Rowe, 2000). In addition to organic causes, asthenospermia (sperm motility less than the WHO cutoff levels) can also be artifactual when spermicides, lubricants, or rubber condoms are used. Occasional clumps of agglutinated sperm are of no consequence. However, more than 10% to 15% of clumping of spermatozoa is indicative of antisperm antibodies (ASAs). ASA is known to reduce sperm motility and cause a peculiar shaking pattern that prevents spermatozoa from penetrating through the cervical mucus. ASA testing must be performed to rule out the presence of antibodies. Other potential causes of asthenospermia include prolonged abstinence periods, genital tract infection, partial ductal obstruction, and varicocele. Loss of motility in all spermatozoa or less than 5% to 10% motility can be caused by ultrastructural defects such as absence of axonemal dynein arms or dead sperm (necrospermia) (McLachlan, 2003). Sperm morphology is the most subjective and most difficult-to-standardize semen parameter. Accurate assessment of morphology is critical in the evaluation of an infertile male because it can be a significant predictor of pregnancy. Normal sperm possess an oval head with a well-defined acrosomal region composing 40% to 70% of the head area. The dimensions of the head are 4 to 5.5 µm in length and 2.5 to 3.5 µm in width. The normal sperm are free from head, midpiece, or tail defects. Head defects include microcephalic head (approximately half the size of a normal sperm head), megalocephalic head (one-and-a-half times the size of a normal sperm head), tapered head, round sperm (missing acrosome), and bicephalic or multicephalic head. Neck defects include no tail or improper tail insertions. Midpiece defects comprise elongated, distended, thin, or bent midpieces. Some of the tail defects commonly noted are short, multiple, bent, or broken tails. One common defect includes coiled tail, which is indicative of osmotic stress (McLachlan, 2003). Sperm morphology is expressed as percentage of abnormal forms present in the semen. The two most common classifications used for the assessment of sperm morphology are the WHO criteria and Kruger’s strict criteria (Table 21–3). When correctable causes of male infertility are not identified, couples with teratozoospermia (<15% normal morphology by WHO method) may be directed to proceed with IVF and ICSI as compared with intrauterine insemination (IUI). Teratozoospermia may occur due to several factors such as fever, varicocele, and stress. Some drugs that affect spermatogenesis are also known to cause morphologic abnormalities. With the advent of ICSI, which requires only one morphologically and functionally normal spermatozoa to fertilize an oocyte, morphologic assessment is losing its significance (Zinaman, 2000). Table 21–3 Sperm Morphology Classification When the motility is reported as less than 5% to 10%, viability testing is recommended because profoundly low motility may indicate dead sperm or necrospermia (McLachlan, 2003). The most common viability assessment involves staining with Eosin Y followed by counter staining with Nigrosin. The viable sperm with its intact cell membrane will not take up the dye and will remain unstained. This test will differentiate necrospermia from immotile sperm secondary to ultrastructural defects such as in Kartagener syndrome and primary cilia dyskinesia. Hypo-osmotic swelling test (HOST) is an alternative method to assess sperm viability. It is based on the principle that viable sperm have intact cell membranes. Exposure of the sperm to hypo-osmotic fluid will cause water to flow into the viable cells seen as swelling of the cytoplasmic space and curling of the sperm tail. Nonviable sperm with nonfunctional cell membranes will not exhibit this effect because they cannot maintain an osmotic gradient. This reproducible and relatively inexpensive test aids in selection of viable sperm for use in IVF or ICSI, especially when no motile sperm are seen in the cryopreserved specimens (Check, 2002). Several nonsperm elements noted on seminal microscopic examination are immature germ cells, epithelial cell, and leukocytes (Branigan et al, 1995; Fedder, 1996). Epithelial cells when present in high numbers are indicative of poor collection. Leukocytes are the most significant nonsperm cellular elements in the semen and are a frequent finding in patients who have unexplained infertility (Branigan et al, 1995). However, in the initial microscopic analysis, the immature spermatozoa may be confused with leukocytes. To confirm the presence of leukocytes, additional testing is therefore required when there are greater than five round cells per high-power field (HPF). Immunocytochemistry is the procedure of choice, but given its expense, it is not widely used in most laboratories. The Endtz test is a reliable alternative because it allows accurate identification of leukocytes that contain enzymes that will react with peroxide and can be visualized with the ortho-toluidine dye (Shekarriz et al, 1995). Initially considered solely as a marker of genital tract infection, contemporary research has shown that leukocytes can be present in the absence of other signs of infection or immune response (Lackner et al, 2006) and that they have intimate links with reactive oxygen species (ROS) (Aitken et al, 1994; Sharma et al, 2001; Saleh et al, 2002; Lackner et al, 2006). The WHO has defined leukocytospermia as levels above 1 × 106 WBC/mL. Studies have shown, however, that ROS levels are elevated even at WBC counts of less than 0.2 × 106/mL, suggesting that much lower levels of leukocytes are pathologic (Sharma et al, 2001; Athayde et al, 2007). In a 12-month follow-up, men who had a negative Endtz test (zero) had a 23.7% chance of initiating pregnancy, whereas leukocytes levels of less than 1 × 106/mL lowered the chances to 15.5% (Athayde et al, 2007). In many andrology laboratories, leukocytospermia determination still has to be requested separately. However, its significance and the ease of determination should place this test among the standard testing that accompanies a basic semen analysis. When leukocytospermia is identified, semen cultures should be performed. Furthermore, red blood cells (RBCs) are also often present in semen. Although small amounts are usually a normal finding, they can be indicative of infection, inflammation, ductal obstruction, or rarely vascular abnormalities. Computer-assisted sperm analysis (CASA) is a semiautomated technique that provides data on sperm density, motility, straight-line and curvilinear velocity, linearity, average path velocity, amplitude of lateral head displacement, flagellar beat frequency, and hyperactivation. It has two distinct advantages over traditional manual analyses: high precision and quantitative assessment of sperm kinematics. Sperm concentration, samplepreparation, and frame rate can, however, affect accuracy of the CASA (Mortimer, 1994). The use of some stains has also affected the accuracy of determining the sperm morphology. Although this technology has theoretic advantages, it has not translated into benefits in clinical practice. This test requires expensive equipment and still requires the active participation of a technician. Therefore at present, these machines are found commonly in andrology laboratories, not in general pathology laboratories, where most of the initial semen analyses are analyzed (Amann and Katz, 2004). Presently, the most important role of CASA is to provide standardized aids in quality control and quality assurance in andrology laboratories, as the emerging use of ICSI has diminished the role of motility assessment in sperm selection (Amann and Katz, 2004). The true litmus test for male fertility remains the ability to cause pregnancy in vivo. Although the semen analysis is used as a surrogate measure of a man’s fertility potential, it is not a direct measure by any means. Clinical research has shown that normal semen analysis may not reflect defects in sperm function (idiopathic infertility), and men with poor sperm parameters still may cause spontaneous pregnancies. Only 50% of infertile men have recognizable causes detectable by the basic semen analysis (MacLachlan, 2003). The presence of several criteria further reinforces the emerging opinion that the current standards do not reflect the true fertility potential of subjects. The current normal values fail to satisfy clinical and statistical standards (McLachlan, 2003; Nallella et al, 2006) and pose the risk of misclassifying a subject’s true fertility status. In fact, 20% of 18-year-olds would be classified as subfertile using the WHO cutoff of 20 × 106 sperm/mL (Andersen et al, 2000). Studies on semen donors with known fertility status have revealed a significant overlap in the sperm characteristics between fertile and subfertile men (Li et al, 2006; Nallella et al, 2006). Guzick and colleagues (2001) in a study of 1461 men found different cutoff levels in sperm concentration (<13.5 × 106 in subfertile and 48 × 106 in fertile men), percent motility (<32% in subfertile and >63% in fertile men), and normal morphology (<9% in subfertile and >12% in fertile men). Nallella and colleagues in 2006 did a similar study (n = 572) and used the WHO and Tygerberg criteria on subjects with known fertility. They noted that there is low sensitivity (0.48) in detecting subfertile subjects using WHO reference values for sperm concentration and low sensitivity (0.83) using Tygerberg criteria for percentage of normal morphology. Among the variables, motility had the least overlap range and gave the best prediction of the subject’s fertility potential. This is in contrast with the earlier study by Guzick and colleagues, where morphology was reported to provide the highest discriminating power in detecting subfertility among all the semen variables. Clearly, each variable alone is neither a powerful sole discriminator nor a predictor of fertility status, and they must be considered in the context of other parameters and the clinical setting. There remains a need for further studies in larger populations and different demographics before a consensus can be reached on the necessity of resetting current values to increase the predictiveness and utility of the semen analysis (Table 21–4). Table 21–4 Characteristics of Normal Semen (World Health Organization, 1999) Cervical mucus is a heterogenous fluid that is composed of 90% water. In order to reach the site of fertilization, the spermatozoon must be able to successfully traverse the cervix and the cervical mucus. In-vitro penetration of spermatozoa through cervical mucus is comparable to in-vivo conditions. The cervical mucus is shown to demonstrate cyclical changes in consistency and to be highly receptive around the time of ovulation. Increase in penetrability is often observed one day before the LH surge. Cervical mucus has been shown to protect the spermatozoa from the hostile environment of the vagina. The penetrability of the spermatozoa through the cervical mucus can be detected by the cervical mucus migration assay. Some methods by which migration can be detected include the postcoital test (PCT). This test can assess cervical environment as a cause of infertility. Accurate timing is crucial because it must be conducted when the cervical mucus is thin and clear just before ovulation. In this test, cervical mucus is examined 2 to 8 hours after normal intercourse. Progressively motile sperm greater than 10 to 20 per HPF is designated as normal. Practical guidelines of the American Society of Reproductive Medicine recommend PCT in the setting of hyperviscous semen, unexplained infertility, or low-volume semen with normal sperm count (Van der Steeg et al, 2004). Medical history and semen analysis can predict PCT results in half of the infertile couples. Poor-quality semen most likely will have poor PCT. Therefore it is not recommended routinely for men who have abnormal semen analyses. Couples who show defective sperm mucus interaction may be advised to proceed with IUI because additional tests are unlikely to affect the management (Guzick et al, 2001). However, abnormal PCT may result from inappropriate timing of the test. Other causes of abnormal PCT include anatomic abnormalities, semen or cervical mucus antisperm antibodies, inappropriately performed intercourse, and abnormal semen. Persistently abnormal PCT in the presence of reasonably good semen parameters should indicate poor cervical mucus quality. The finding of good-quality mucus with nonmotile spermatozoa or immobilized sperm demonstrating shaking motion should lead to the evaluation of both partners for the presence of antisperm antibodies. Although it has fallen out of favor, this test may be useful in patients who are unable or unwilling to produce an ejaculate. The sperm penetration assay (SPA) or the hamster egg penetration assay (HEPT) determines the functional capacity of the spermatozoa necessary to fertilize an oocyte. It is based on the principle that normal spermatozoa can bind and penetrate the oocyte membrane, which is a prerequisite for the fusion of sperm and the oocyte. Zona pellucida is the outermost layer protecting the cytoplasm of the oocyte. It plays an important role in the fertilization process and is shown to be the only physiologic inducer of acrosome reaction. Sperm binds to the species-specific receptor, ZP3, which is found on the zona pellucida of the oocyte. The zona-free hamster oocytes, in which the zona pellucida is stripped, are used to allow cross-species fertilization. Human sperm penetration assay with zona-free hamster eggs determines the ability of sperm to successfully undergo capacitation, acrosome reaction, membrane fusion with oocytes, and chromatin decondensation. The assay is performed by incubating zona-free hamster oocytes in sperm droplets for 1 to 2 hours. The oocytes are examined microscopically for sperm penetration. Penetrations are indicated by swollen sperm heads within the oocyte cytoplasm. Normally, 10% to 30% of ova are penetrated (WHO, 1999). Oligozoospermic and severely teratospermic men have a higher number of defective sperm-zona pellucida interactions, which may account for their low fertility potential in both spontaneous and IVF pregnancies (Liu and Baker, 2004). Despite its low predictive power, SPA is correlated positively with spontaneous pregnancy outcomes (Corson et al, 1988). Sperm capacitation index (SCI) is a variant of the SPA test, assessing the mean number of penetrations per ovum. ICSI has been recommended for couples with an SCI less than 5 instead of standard IVF procedures (Ombelet et al, 1997). Compared with SPA, the zona binding test uses oocytes that failed to fertilize in IVF clinics. The need for human oocyte supply, however, remains a limitation to the use of this test. Approximately 10% of infertile men will present with ASA as compared with 2% of fertile men (Guzick et al, 2001). Sperm parameters are often normal in men with ASA (Munuce et al, 2000). Hence it has been suggested to be tested routinely in all men undergoing infertility work-ups (McLachlan, 2003). Excessive sperm agglutination or an abnormal PCT can suggest the presence of ASA. The direct ASA test detects sperm-bound immunoglobulins. Indirect testing detects the biologic activity of circulating ASA. False positives can result from nonimmunologic factors (Francavilla et al, 2007). Because only antibodies present on the sperm surface are clinically significant, most investigators prefer direct assays that determine sperm-bound antibodies instead of indirect detection of serum antisperm antibodies. IgG-MAR (mixed antiglobulin reaction) and Sperm MAR are recommended screening tests that are economical and readily available. Immunobead Test (IBT), which measures IgG, IgA, and IgM, may be additionally recommended when either of the previous tests gives a positive result in order to determine if IgA are bound to sperm surface. Acceptable normal values by WHO (1992) standards include less than 10% (IgG MAR) or 20% (IBT) of spermatozoa with adherent particles. Clinical implications of ASA on male infertility are varied. A weakly positive IgG MAR/IBT in men who have low motile sperm rules out immunologic factors, and no further testing is necessary (Francavilla et al, 2007). ASA are present in 34% to 74% of vasectomized men and persist in 38% to 60% after vasectomy reversal (Broderick et al, 1989; Francavilla et al, 2007). Routine ASA testing is not recommended in this setting because it is of uncertain significance and usually does not affect the decision to do a vasectomy reversal. There are conflicting reports regarding ASA levels after orchidopexy for cryptorchidism (Mirilas et al, 2003). In genitourinary infections, ASA is thought to be a consequence of the inflammatory process rather than cross reactivity to the microorganism (Francavilla et al, 2007). The decision to proceed with IUI versus ICSI in immunologic infertility can be aided by a zona pellucida (ZP) test. If the sperm exhibit inability for ZP binding, ICSI is the procedure of choice. Presently, flow cytometry techniques are being developed to quantify ASA in individual spermatozoa (Shai et al, 2005). These techniques are also being explored to identify sperm surface antigens for possible immunocontraceptive development.
Introduction: Definition and Demographics of Infertility
History and Review of Systems
Physical Examination
Genital Examination
Laboratory Evaluation of Male Infertility
Semen Analysis
Collection and Timing
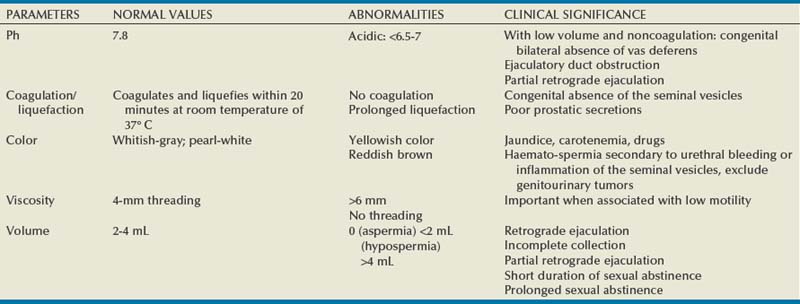
Macroscopic Assessment
Microscopic Assessment
Sperm Agglutination
Count and Concentration
Motility
Morphology
WORLD HEALTH ORGANIZATION 3RD
STRICT WORLD HEALTH ORGANIZATION 4TH (KRUGER)
Normal reference range
>30%
>14%
Head
Shape
Oval
Oval, smooth borders
Acrosome
40%-70% of head surface
40%-70% head surface
Size
4-5.5 mm length
2.5-3.5 mm width
Length/width 1.5-1.75
3-5 mm length
2-3 mm width
Vacuoles
<20% head area
Up to 4
Midpiece
Shape
Straight regular outlined
Axially arched
Slender, straight, regular outline
Axially arched
Size
< 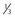 of head area
of head area
<1 mm wide
Length 1.5 × head
Cytoplasmic droplet
< 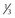 of head area
of head area
< 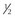 of head area
of head area
Tail
Appearance
Slender, uncoiled
Uniform, uncoiled
Width
Thinner than midpiece
Length
>45 mm
10 × head
Viability
Nonsperm Cells
Computer-Assisted Sperm Analysis
Limitations of Semen Analysis
Color
White, opalescent
Specific gravity
1.028
pH
7.35-7.50
Volume
2-6 mL
Count
2 × 106 spermatozoa/mL or more
Motility
≥50% motile (grades A + B) or 25% with progressive motility (grade A)
Morphology
>30% sperm with normal morphology
Viability
≥50% viable sperm
Pus cells
<1 × 106/mL of semen
Sperm Function Assessment
Sperm-Mucus Interaction/Postcoital Test
Sperm Penetration Assays/Sperm Zona Binding Tests
Advanced Semen Testing
Antisperm Antibody Testing
Male Infertility