Chapter 97D Liver transplantation for hepatocellular carcinoma
Overview
Cancer of a solid organ is an uncommon indication for organ transplantation, but hepatocellular carcinoma (HCC) is an important exception to that general rule (see Chapter 97A). HCC is the most common tumor treated with whole-organ transplantation, and it is listed as the primary indication in approximately 15% to 20% of liver transplantations. The existing treatment options for HCC include hepatic resection, local ablation, or liver transplantation, and in the last decade, transplantation has become the therapy of choice for early-stage disease. The literature lacks randomized controlled trials regarding the treatment of HCC; most centers therefore base their practice guidelines on local experience, retrospective data, theoretic decision analyses, and the availability of treatment options.
Ever since the first liver transplantation was performed in a patient with HCC in the 1960s, recurrence has been a concern. Prior to the late 1990s, liver transplantation for HCC carried high recurrence rates, and the 2-year survival was only about 30%. The poor outcomes were due to the consensus at that time to treat smaller tumors with liver resection and more advanced disease with liver transplantation. Discouraging results plagued liver transplantation for HCC, until it was noted that subgroups of patients with smaller tumors who underwent transplantation achieved better outcomes (Pichlmayer, 1993).
A group from Milan, Italy, conducted a prospective trial, published in 1996, in which patients with smaller tumors achieved 4-year survival rate of 75%, equivalent to the survival for non-HCC patients who underwent transplantation (Mazzaferro et al, 1996), and similar results were seen in the United States (Venook, 1993). Resultant changes in patient selection, dictated by these Milan criteria, allowed similar results to be reproduced at other centers (Jonas et al, 2001; Llovet et al, 1998), and the United Network of Organ Sharing (UNOS) adopted these criteria in 1998 and gave priority for transplantation to HCC patients. This priority, coupled with the rise in patients with hepatitis C virus (HCV), who frequently develop HCC, fueled the rapid expansion of this indication for transplantation. This chapter will discuss liver transplantation for HCC, concentrating on its unique pathophysiology and how it relates to diagnosis and dictates therapy.
Hepatocellular Carcinoma
HCC is the fifth most common cancer and the most common primary liver tumor (see Chapter 80). This disease develops in the setting of cirrhosis in more than 90% of cases (Fattovich et al, 2004). The risk of HCC development in patients with cirrhosis from any cause is about 2.0% to 6.6% per year versus 0.4% for patients with viral hepatitis without cirrhosis (Benvegnu et al, 2004; Degos et al, 2000; Ikeda et al, 1993a; Llovet et al, 2003; Sangiovanni et al, 2004; see Chapter 64, Chapter 70A, Chapter 70B ).
The incidence of HCC has been rising since the late 1990s (Bosch et al, 2004; Deuffic et al, 1998; El-Serag & Mason, 1999; Parkin et al, 2001; Stroffolini et al, 1998; Taylor-Robinson, et al, 1997), a trend that is expected to continue for at least the next 2 decades because patients who were infected with HCV 20 to 30 years ago are currently living with cirrhosis and are aging (El-Serag, 2004). In addition, the epidemic of obesity and resultant nonalcoholic fatty liver disease (NAFLD) can be expected to generate cirrhotic patients at risk for the foreseeable future (see Chapter 65). Currently, only the incidence of thyroid cancer is rising faster than the incidence of HCC in the United States (Fattovich et al, 2004). It is hoped that the introduction of vaccination for HBV will decrease the incidence of HCC, an effect that has been seen already in Taiwan (Chang et al, 2009).
Biology of Hepatocellular Carcinoma
An understanding of the biology of HCC is critical to assist in clinical decision making about surveillance and treatment. Although the molecular biology of HCC remains to be fully elucidated, the close association between cirrhosis and the development of HCC is clear (Miyazawa et al, 2003; see Chapter 8C). Viral hepatitis, chronic alcohol use, and NAFLD are the most common causes of cirrhosis in the Western world (see Chapters 64, 65, and 70A). NAFLD, an unfamiliar diagnosis as recently as the early 1980s, is now present in 17% to 30% of Americans (Farrell & Larter, 2006); that progresses to steatohepatitis in 32% to 37% (Fassio et al, 2004; Harrison et al, 2003; Hui et al, 2003) and then to cirrhosis in 5% to 24% (El-Zayadi, 2008; Hui et al, 2003). The development of cirrhosis in patients with NAFLD is driven by repetitive liver injury from lipid peroxidation, fatty acid toxicity, mitochondrial impairment, and oxidative stress.
Viral infection with HBV and HCV lead to cirrhosis by different mechanisms. HBV induces transgene activation, oncogene transcription, and loss of tumor suppressor genes as a result of viral DNA integration into the host genome (Brechot et al, 1980; Bressac et al, 1990; Di Bisceglie, 2000; Kim et al, 1991). As many as 30% of patients with chronic HBV without cirrhosis will develop HCC (Zhou et al, 2001). HCV does not integrate into the host genome but rather leads to chronic inflammation through continued viral replication (Sherlock, 1994). This rapid hepatocyte turnover in the presence of oxidative stress drives the development of multiple dysplastic nodules.
Unlike what has been found in some other cancers, no sequential progression of genetic defects has been identified that leads to the development of HCC. Instead, a variety of genetic alterations are commonly seen, and they have been broadly grouped into events that occur either early or late in the development of a tumor. Genetic alterations that lead to inhibition of the insulin-like growth factor II receptors are considered early mutations (Yamada et al, 1997). Loss of heterozygosity of a variety of genes, often of tumor suppressor genes such as TP53, is a late event in the development of neoplasia (Buetow et al, 1989; Fang et al, 2000).
Clinically Relevant Aspects of Hepatocellular Carcinoma Pathogenesis
First, HCC most commonly develops in the context of cirrhosis, and the biology of HCC is related directly to the environment found in the cirrhotic liver. Repeated insult causes chronic inflammation driven by tumor necrosis factor (TNF)-α; transforming growth factor (TGF)-β and interleukin (IL)-1 from Kupffer cells; endothelial cells; and hepatocytes. This inflammation stimulates nodular regeneration with bridging fibrosis. Rapid cellular turnover in this regenerative environment leads to low-grade dysplastic nodules that do not contain atypia and high-grade dysplastic nodules that do. Eventually, a small percentage of these high-grade dysplastic nodules will go on to develop microscopic foci of HCC before becoming frank carcinoma (Rocken & Carl-McGrath, 2001). Recent evidence suggests that tumor cells may even secrete IL-6 and TNF-α, directly contributing to the inflammatory microenvironment by activating Toll-like receptors (Kim et al, 2009).
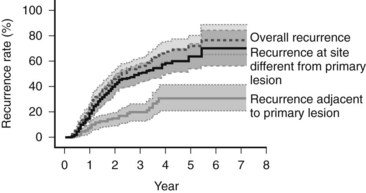
Because of the diffuse nature of the injury, multiple tumors can develop at the same time. If a focus of HCC is removed, recurrence most often happens at a distant site (Koike et al, 2000; Fig. 97D.1). This second tumor at the distant site is most likely a second primary tumor. As most of the second lesions are found within a few years of treatment of the primary lesion, the tumor was most likely present when the first tumor was treated but was too small to be detected. The occurrence of synchronous and metachronous lesions in the same organ suggests the entire cirrhotic organ has the potential for neoplastic conversion, much like the genetic defect in the colon in familial adenomatous polyposis, which leads to multiple cancers; this situation is referred to as a field defect.
Secondly, there is a clinically relevant difference in blood supply between foci of HCC and low-grade dysplastic nodules, which derive most of their blood supply from the portal vein. Histopathologic evaluation of high-grade dysplasia and HCC demonstrates the tumor being fed by branches of the hepatic artery that are not paired with the other structures of the portal tract, a process called arterial recruitment, or neoangiogenesis. The resulting hepatic artery dominance in blood supply produces the characteristically hyperdense appearance of HCC that is used to identify tumors during the arterial phase of contrast imaging (Matsui, 2004; Willatt et al, 2008), and it allows for treatment via selective hepatic artery embolization.
Lastly, HCC is known to invade vascular structures, most commonly the portal vein but also hepatic veins and, less commonly, the biliary tree. The development of vascular invasion appears to be a key step in the metastatic potential of the tumor, and the risk of portal venous invasion seems to be directly related to size and differentiation status of the tumor (Esnaola et al, 2002). Venous invasion is such a strong predictor of metastatic disease that tumor size and tumor number can be thought of as surrogate markers for vascular invasion.
Hepatocellular Carcinoma Screening
HCC presenting as symptomatic disease has a 0% to 10% 5-year survival rate (Llovet et al, 2003), so a screening program that identifies early disease can have a major impact on treatment outcomes. HCC meets the World Health Organization criteria for performing screening (Meissner et al, 2004), and a randomized, controlled clinical trial showed that screening for HCC reduces mortality (Zhang et al, 2004). An effective screening program evaluates patients at risk of developing disease, using a highly standardized and relatively inexpensive test with a high sensitivity. A screening program is used if the cost of screening a large population is less than the benefit of early detection of the disease in a small number of individuals in that population. Several decision-analysis and cost-efficiency models for HCC screening suggest the ability of a screening program to decrease mortality while remaining cost-effective depends on the inclusion of patients with an incidence of HCC of at least 1.4% to 1.5% (Arguedas et al, 2003; Lin OS, et al, 2004; Sarasin et al, 1996). Patients who should be included in a screening program can be found in Box 97D.1. Further information about the incidence of HCC in cirrhosis from nonalcoholic steatohepatitis (NASH), α1-antitrypsin deficiency, and autoimmune hepatitis is required, but most centers include these patients in their screening population.
Box 97D.1 Populations with Hepatocellular Carcinoma Incidence Sufficiently High for Inclusion in Surveillance Programs
Traditionally, the most commonly used serum tumor marker to detect HCC was α-fetoprotein (AFP), a protein produced by normal fetal hepatocytes, and by tumor cells, but not by healthy, mature hepatocytes. AFP has traditionally been used for diagnosing HCC, predicting outcome after resection, and screening for recurrence postoperatively (Tan et al, 2003). The normal range of AFP in the serum is 10 to 20 ng/mL; an absolute value of 20 ng/mL was used commonly as the screening cutoff; however, using this cutoff yields a sensitivity of only about 64%, making it a poor screening test (Sherman et al, 1995). Moreover, normal AFP levels vary widely across ethnic backgrounds (Soresi et al, 2003), and the test has a lower positive predictive value in patients with acute viral hepatitis or cirrhosis from a viral cause (Nguyen et al, 2002; Soresi et al, 2003).
In patients with a liver mass, a concomitant serum AFP greater than 200 ng/mL carries a very high positive predictive value (Sherman et al, 1995), but because of its unacceptably low sensitivity and specificity, the American Association for the Study of Liver Disease (AASLD) recommended that serum AFP no longer be used as a screening tool, although it still plays a diagnostic role (Bruix & Sherman, 2005). Using an absolute value greater than 400 ng/mL is considered diagnostic regardless of the patient’s ethnic background or the cause of their liver disease. Research is ongoing to identify better serum markers for screening, diagnosis, and prognostication, and possible candidate compounds include AFP-L3 and des-γ-carboxy prothrombin (Bruix & Sherman, 2005; Grazi et al, 1995; Izuno et al, 1995; Koike et al, 2001; Marrero et al, 2003; Tsai et al, 1990).
Ultrasound (US) is the most widely used radiologic screening tool. It has a sensitivity of 65% to 85% and specificity over 90% when used as a screening test (Bolondi et al, 2001). Although the performance of US in cirrhotic patients is not as well defined (Bruix et al, 2001; Chen et al, 2002; Larcos et al, 1998; Sherman, 1999), and the test is operator dependent, US remains superior to any widely available serologic screening test. A large, prospective, randomized controlled study done in China suggests that surveillance of high-risk individuals with US and serum AFP testing every 6 months reduces HCC-related mortality by close to 40%, even though patients in that trial were offered only resection but not transplantation. One of the limitations of this study is that screening compliance was only approximately 60%, which may have dampened the reduction in mortality (McMahon, 2009; Zhang et al, 2004). Combined AFP and US surveillance increases sensitivity, but it raises the cost, and using both tests increases the rate of false-positive results from 5% for AFP alone to 7.5% for combined screening (Zhang & Yang, 1999). It is clear that further randomized controlled trials are needed, but based on the available information, the AASLD recommends screening patients at risk for HCC with US alone every 6 to 12 months (Bruix & Sherman, 2005). Evaluation of serum AFP can be added to US in the event of concerns regarding operator influence, but the use of AFP as the lone surveillance tool is discouraged.
Most centers use a 6-month screening interval, but this remains controversial. No evidence suggests that a 6-month interval improves outcome compared with a 12-month interval (Santagostino et al, 2003). The surveillance interval was derived from tumor growth rates, so the interval does not need to be shortened for patients who have a very high risk of developing HCC.
A nodule that is 2 cm or larger and hypervascular with portal venous washout on a single dynamic imaging study, or a 1- to 2-cm nodule on two such confirmatory studies, is treated as HCC. If the results are contradictory, a biopsy of the lesion should be obtained (Bruix & Sherman, 2005). If the lesion is larger than 2 cm and has a typical appearance on a single dynamic imaging study, or if it is found in concert with a serum AFP level above 200 ng/mL, biopsy is not required. Biopsy should be performed on any 1- to 2-cm lesion in any noncirrhotic patient has who has risk factors for the development of HCC. Biopsies should be read by expert pathologists, and patients with negative biopsy results should undergo US or CT scanning every 3 to 6 months and should be rebiopsied if the characteristics of the lesion change (Bruix & Sherman, 2005). Biopsy results that suggest a high risk for progression to HCC include dysplastic nodules, both low and high grade (Hytiroglou et al, 2007); small and large cell change, formerly called small cell dysplasia and large cell dysplasia (International Working Party, 1995; International Consensus Group for Hepatocellular Neoplasia [ICGHN], 2009); and dysplastic foci, defined as atypia outside of a dysplastic nodule (ICGHN, 2009).
Another commonly used radiologic surveillance tool is CT scanning (Kobayashi et al, 1985; Miller et al, 1994; Takayasu et al, 1990). Although the use of CT scanning is becoming commonplace, the radiation exposure to a patient from screening every 6 to 12 months is considerable. The use of CT for diagnosis and staging has been defined, but the performance of CT as a screening test has not been described. Finally, CT scan is the diagnostic test used to confirm HCC, and the screening test should be different from the confirmatory test.
MRI with gadolinium-based or iron oxide–based contrast has not been widely studied as a screening test, but there are reports that this modality is 81% sensitive and 85% specific for HCC compared with 68% and 93% for contrast-enhanced helical CT scanning (Colli et al, 2006). Moderate signal strength on T2-weighted images is specific for HCC, because dysplastic and regenerating nodules are not hyperintense on T2—unless they have infarcted, which can occur. Delayed venous phase hypointensity with concomitant delayed enhancement of the rim of the lesion are also features specific for HCC (Freeny et al, 2003). Similar to CT scanning, characterization of small nodules remains difficult, as MRI is only 50% to 80% sensitive for lesions 2 cm or smaller and only 4% to 33% sensitive for lesions 1 cm or smaller (Willatt et al, 2008); this is due in part to the fact that lesions 1 cm or smaller are frequently isointense on T2 (Krinsky, 2004). It remains to be seen whether contrast-enhanced MRI is cost-effective as a screening tool for HCC, but it may prove to be marginally superior to CT scanning for characterization of known hypervascular lesions.
Treatment Options
HCC is not responsive to traditional cytotoxic chemotherapeutic agents. Recently, a Phase III clinical trial showed that sorafenib, an oral multikinase inhibitor of the vascular endothelial growth factor receptor, the platelet-derived growth factor receptor, and Raf kinase prolonged the median survival (10.7 vs. 7.9 months) and the time to radiologic progression (5.9 vs 2.8 months) in patients with advanced HCC versus placebo (Llovet et al, 2008). Given this modest improvement, local ablation/embolization, hepatic resection, and liver transplantation are still the best options available for patients with early stage disease to achieve a cure.
Treatment decisions should be based on three factors: 1) extent of local disease, 2) underlying liver disease, and 3) local organ availability. Patients whose liver disease is Child-Turcotte-Pugh (CTP) class A who have early-stage HCC are candidates for treatment with the goal of long-term survival free of HCC. Unfortunately, because of the advanced biologic activity of the cancer, 80% to 90% of patients will present at a stage too advanced for surgical resection or transplantation (Llovet et al, 2004). Those patients with advanced disease or patients with poor functional status should be offered sorafenib, locoregional therapies, or palliative care.
In the rare patient without cirrhosis, no evidence of venous invasion or metastatic disease, and tumor burden limited to one lobe, resection should be considered. Conversely, resection of part of a cirrhotic liver invariably leaves scattered dysplastic nodules behind. The tumorigenic cytokine milieu that bathes the remaining dysplastic nodules puts the patient at risk for the development of a second primary tumor. In addition to these de novo tumors, some researchers have suggested recurrence is more frequently the result of microscopic dissemination of the primary tumor at the time of treatment (Chen et al, 2000; Morimoto et al, 2003). Astoundingly, the risk of developing recurrence after resection for HCC in cirrhotic patients is about 35% at 1 year (Lu et al, 2009), 40% to 50% over 3 years (Koike et al, 2000; Jaeck et al, 2004; Sakon et al, 2000), and as high as 70% at 5 years (Bismuth et al, 1999; Llovet et al, 1999; Mazzaferro et al, 1996; Minagawa et al, 2003), making the history of a previous HCC the greatest risk factor in the development of a new primary HCC.
It is this high risk of recurrence that has driven liver transplantation as a method to remove the precancerous liver to prevent the development of subsequent lesions. The results of liver transplantation have improved dramatically in the last 20 years, in large part because of better patient selection, and currently the recurrence rate ranges from 11% to 18%. The mortality rate after liver resection for HCC is 1% to 7%, and the major cause is decompensated liver failure (Fong et al, 1999; Grazi et al, 2001; Hanazaki et al, 2000; Hsia et al, 2000; Makuuchi et al, 1998; Poon et al, 2001; Takenaka et al, 1996; see Chapter 90F). Given the risks of HCC recurrence and postoperative liver failure following resection, some controversy exists regarding the best treatment strategy in patients with limited disease and adequate hepatic function. Although sufficient hepatic reserve can usually be determined, no method is currently available to determine whether a patient will be among the 60% to 70% to recur within 5 years, or if they will remain recurrence free.
A decision analysis from 2000 compared primary resection and salvage transplantation for recurrence versus primary liver transplantation in patients with small tumors (Majno et al, 2000). Theoretic decision models use multiple variables, so the weight of any single variable is difficult to determine, but the authors concluded that wait times shorter than 6 months favored liver transplantation, and those longer than 12 months favored primary liver resection.
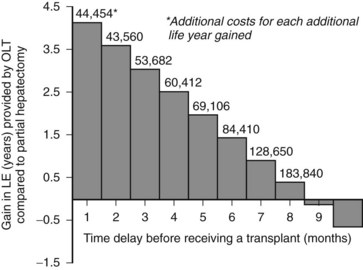
Another decision analysis compared the projected survival benefit from liver transplantation when compared to resection in patients with small HCCs (Sarasin et al, 1998). The authors noted that the magnitude of the survival benefit relied heavily on the amount of time spent on the waiting list. They concluded that if the waiting period exceeded 6 to 10 months, the survival benefit gained from removal of the field effect is overwhelmed by the risk of progression while waiting, and resection becomes the preferred therapy (Fig. 97D.2). Although this analysis did not include the costs or clinical effects of locoregional therapy, it offers insight into how extended waiting times increase the relative benefit of resection, transarterial chemoembolization (TACE), and radiofrequency thermal ablation (RFA; see Chapters 83 and 85A).
The possibility of mitigating the risk of progression with a locoregional therapy while awaiting a transplantation has been suggested. Whether locoregional therapy offers a measure of disease control while awaiting a liver transplantation is not clear. Maddala and colleagues (2004) examined 54 patients with HCC treated with an average of three TACE sessions prior to transplantation at a median of 211 days. Eight patients dropped off the waiting list, two for non-HCC reasons and six from HCC progression. Of the six with progression, two developed extrahepatic metastases, two developed portal vein invasion, and two had intrahepatic progression. Interestingly, these events all occurred within 4 months of listing. The short period of time between initiation of therapy and discovery of disease progression would suggest the disease had spread much earlier, and neither resection nor transplantation would have been helpful.
In a small study of 20 patients (median of two treatments, mean time to transplantation of 343 days), Hayashi and colleagues (2004a) found that seven patients whose tumors were within Milan guidelines dropped off the waiting list: two for causes unrelated to HCC, one for decompensation, and the other five for increased intrahepatic disease. The time to exclusion for HCC was less than 6 months in four of five patients, and no patients developed HCC after transplantation at a mean follow-up of 2.9 years.
RFA has also been reported to provide good results in patients who undergo subsequent transplantation. Mazzaferro and colleagues (2004) reported 50 patients who underwent a single ablation session and then waited an average of 9.5 months before transplantation. Apparently, there were no waiting list dropouts, and at a median follow-up of 22 months, only two patients had cancer recurrence. Lu and colleagues (2005) reported 52 patients who underwent RFA, most frequently in isolation (mean sessions 1.46). After 12 months, three patients had dropped out at a mean of 11 months, and two patients had extrahepatic disease; in all, 41 patients underwent transplantation, and none had HCC recurrence at a mean follow-up of 15 months. For small tumors, it has been suggested that RFA may be equivalent to resection for long-term disease control, with a lower risk of postoperative liver decompensation (Shiina, 2009), but further trials with long-term outcome data are required.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree