Chapter 90F Liver resection in cirrhosis
Overview
Hepatocellular carcinoma (HCC) is the main indication for hepatectomy in the cirrhotic liver. The vast majority of HCC in patients with chronic liver disease (CLD) is related to hepatitis B or C viral infection, chronic alcoholic consumption, and metabolic syndrome (see Chapter 70A, Chapter 70B, Chapter 80 ). Other malignant tumors developed in CLD include intrahepatic cholangiocarcinoma (see Chapter 50A) and liver metastases (see Chapter 81A, Chapter 81B, Chapter 81C ; Iascone et al, 2005; Uchiyama et al, 2011; Welzel et al, 2007;). Some benign tumors—including regenerative nodules, hemangiomata, and focal nodular hyperplasia (FNH)—can be discovered in cirrhotic liver (Brancatelli et al, 2001a, 2001b; Libbrecht et al, 2006; Luciani et al, 2007; see Chapter 79A, Chapter 79B ).
Although liver transplantation (LT) offers the best chance of cure in patients with early stage HCC (Mazzaferro et al, 2009; see Chapter 97A), the persistence of graft shortage in many countries had led some authors, including those in our group, to advocate hepatic resection as the first-line treatment for HCC in cirrhotic patients with preserved liver function (Belghiti et al, 2008; Cherqui et al, 2009; Del Gaudio et al, 2008; Poon et al, 2002a). Liver resection is an effective treatment for HCC under appropriate conditions, but impaired liver regeneration, altered texture of liver parenchyma, portal hypertension, and collateral circulation make liver resection in cirrhotic patients challenging.
In the 1990s, operative mortality in patients with normal livers was 1%, compared with 8% among patients with diseased parenchyma (Belghiti et al, 2000; see Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90D, Chapter 90E ). The higher risk associated with liver resection in patients with diseased parenchyma results from increased operative bleeding and postoperative complications, with liver failure mainly as a result of impaired liver regeneration in cirrhosis (see Chapter 5); however, with appropriate attention to preoperative patient selection, improved liver function assessment, better understanding of the segmental liver anatomy with more accurate imaging studies, advanced operative techniques, and better perioperative management, hepatectomy now can be performed in the cirrhotic liver with acceptable operative morbidity and mortality in major centers (Dahiya et al, 2010; Ferrero et al, 2005; Imamura et al, 2003; Wei et al, 2003). This chapter describes general surgical aspects and risks of liver surgery in patients with liver cirrhosis.
Preoperative Assessment
The improvement in the outcomes of liver resection in cirrhotic patients is mainly the result of good preoperative evaluation and appropriate patient selection (Ikai et al, 2007). A systematic and careful assessment of the patient’s general medical fitness, the tumor extent and stage, the underlying liver function, and the volume of the anticipated future liver remnant (FLR) are critical in ensuring proper patient selection (see Chapter 2, Chapter 70A, Chapter 70B ).
General Status of Patients
Attention to the general medical fitness of patients is paramount in selecting patients with HCC for hepatic resection, especially in cirrhotic patients, who are at higher risk of postoperative complications. Comorbid illness is a major predictor of the mortality of patients undergoing hepatectomy (Wei et al, 2003). Severe comorbid illnesses, such as congestive heart failure and chronic renal failure, are definite contraindications for hepatectomy, although patients with less severe comorbid illnesses may still benefit from hepatic resection, if accompanied by meticulous perioperative care. The impact of diabetes mellitus, which is a common comorbid illness in cirrhotic patients, is debated (Gedaly et al, 2009). It has been shown that postoperative morbidity and mortality after hepatectomy in patients with diabetes mellitus were similar to those of patients without diabetes mellitus, as long as optimal perioperative control of blood glucose levels and appropriate postoperative care were provided (Poon et al, 2002b).
Tumor Status
The assessment of the tumor extent included a triple-phase (early vascular or arterial phase, portal phase, and delayed phase) helical computed tomography (CT) of the thorax and the abdomen (see Chapter 16). The liver is studied using thin slices acquired during the unenhanced phase and during the arterial, portal, and late or equilibrium phase after contrast administration. HCC is hypervascular during the early arterial phase and hypovascular in late phase (“washout”). Magnetic resonance imaging (MRI) is the modality of choice for imaging when contrast agents are contraindicated, better lesion characterization is needed, or the anatomic relationship between tumor and major vascular or biliary structures requires further delineation (see Chapter 17).
The usual criteria for hepatic resection with regard to the tumor status include absence of extrahepatic metastasis and tumor thrombus in a main portal vein or in the inferior vena cava (see Chapter 80). Large tumor size alone should not be considered a contraindication for hepatic resection. Despite the technical problems encountered with large tumors, liver resection for large HCCs has been shown to be safe (Kosuge et al, 1993; Pawlik et al, 2005a, 2005b; Ramacciato et al, 2010; Yang et al, 2009). In a series of 300 patients who underwent partial hepatectomy for HCCs larger than 10 cm, the 30-day mortality rate was 5% (Pawlik et al, 2005b). Because cadaveric orthotopic liver transplantation (OLT) and radiofrequency ablation (RFA) are contraindicated in these patients, surgical resection remains the treatment of choice for large HCCs.
HCC associated with biliary invasion should not be considered a contraindication to surgical resection, if curative resection can be achieved (Hanaoka et al, 2008; Lee et al, 2006; Peng et al, 2005). Multifocal disease generally is a contraindication to surgical resection, but in some patients with good liver function, resection can be reconsidered after clearance or stabilization of contralateral liver nodules by chemoembolization or RFA (Ishizawa et al, 2008; Liu et al, 2003; Ng et al, 2005; Ramacciato et al, 2010).
HCCs with major portal or hepatic vein involvement represent a technical and oncologic challenge. Patients with HCC that involves hepatic veins or vena cava have a high rate of pulmonary metastases (Ikai et al, 2003), although it has been shown that in a selected group of patients with normal liver function and excellent general status, extensive liver resection with removal of the vascular thrombus can achieve favorable survival results (Le Treut et al, 2006; Minagawa et al, 2001; Poon et al, 2003; Shi et al, 2010). Extension to surrounding structures, such as the diaphragm, does not represent a contraindication, provided that negative resection margins can be attained.
Tumor recurrence represents the major drawback after curative liver resection and is the most common cause of treatment failure. Within 5 years of resection, recurrence in the liver remnant occurs in about 50% to 70% of cases (Huang et al, 2010; Poon et al, 2000; Shi et al, 2007). The rate of repeated resection, ranging from 10% to 30%, depends on the underlying liver status, pattern of recurrence, and extent of first resection; lower rates are seen in series with a high proportion of major resection during the first hepatectomy, and repeat hepatectomy has been proven to be a safe and worthwhile procedure with a low mortality rate and an acceptable 5-year survival (20% to 57%) (Minagawa et al, 2003; Poon et al, 1999; Tsujita et al, 2010; Wu et al, 2009).
Assessment of Liver Function Reserve (See Chapters 2 and 70B)
Child-Turcotte-Pugh Classification
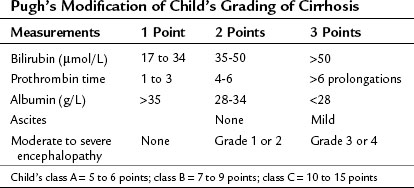
In most of Western centers, preoperative liver function is assessed using the Child-Turcotte-Pugh (CTP) classification, which incorporates variables related to conventional liver function and portal hypertension. This classification is easy and remains very accurate in its assessment of the surgical risk (Table 90F.1). According to this system of classification, major resection is performed only in CTP class A patients, limited resection of small superficial tumors is sometimes performed in class B patients, and no resection, regardless of the extent, is considered in class C patients; however, this classification is not enough to predict surgical risk in some patients, because two parameters are subjective—ascites and encephalopathy—and even within the class A group with apparently good liver function, some degree of portal hypertension and/or hepatic dysfunction may be present, and the risk of liver surgery may be underestimated. Therefore more sophisticated quantitative liver function tests or volumetric assessments are needed to predict the operative risk (Durand & Valla, 2005).
Indocyanine Green Test
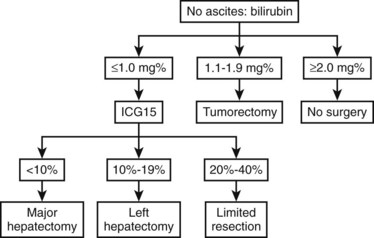
Most Eastern centers use more sophisticated quantitative liver function tests that include indocyanine green (ICG) clearance (Imamura et al, 2003), galactose elimination capacity (Redaelli et al, 2002), and a lidocaine test (Ravaioli et al, 2003) to predict the risk of postoperative liver failure in patients with cirrhosis. The Hong Kong group showed in a multivariate analysis that of the many liver function tests, ICG retention at 15 minutes to 20% was the best test for predicting hospital mortality after major hepatectomy for HCC (Lau et al, 1998; Lee & Hwang, 2005). Imamura and Makuuchi proposed a decision tree based on combined parameters from the CTP classification (ascites and bilirubin) and ICG retention test (Imamura et al, 2005; Fig. 90F.1). Based on this strategy, liver resection is contraindicated in patients who have elevated bilirubin (>2 mg/dL) or ascites. The extent of liver resection is then based on the ICG retention test at 15 minutes (see Table 90F.1), and major liver resection is proposed only in patients with normal bilirubin and ICG levels below 10%. Adopting this decision strategy, the authors reported only one mortality among the 685 patients who underwent liver resection for HCC (Imamura et al, 2005).
Model for End-Stage Liver Disease Score
The Model for End-Stage Liver Disease (MELD) score uses only objective variables calculated from the international normalized ration (INR), serum total bilirubin (mg/dL), and serum creatinine (mg/dL). This system was initially created to predict survival in patients with complications of portal hypertension undergoing elective placement of transjugular intrahepatic portosystemic shunts (TIPS) (Malinchoc et al, 2000). The MELD score was validated subsequently as an accurate predictor of survival among different populations of patients with advanced liver disease (Kamath et al, 2001). The most frequent use of the MELD score has been in the allocation of organs for patients awaiting liver transplantation (Freeman et al, 2005; Wiesner et al, 2001). Regarding liver resection in cirrhotic patients, the MELD score was only retrospectively studied. Cirrhotic patients who underwent liver resection for HCC with a MELD score greater than 8 had a higher risk of death, morbidity, and impaired long-term survival (Cucchetti et al, 2006; Delis et al, 2009).
Transaminase Levels
Postoperative mortality has been shown to be higher in a cohort of 285 patients who underwent hepatectomy for HCC when histologic evidence of cirrhosis and active hepatitis versus cirrhosis alone was found (Eguchi et al, 2000). Although the presence of high serum transaminase levels does not always correlate with hepatitis and could be associated with intratumoral necrosis in a huge tumor, increased complication and death rates have been reported in those patients with levels of elevated transaminases (Haber et al, 1995; Noun et al, 1997). Patients with an aspartate aminotransferase level greater than 100 IU/L or alanine aminotransferase at least twice the normal level are considered poor candidates for major hepatic resection. In this setting, preoperative biopsy of the nontumorous liver should be performed to exclude active underlying liver parenchymal disease.
Portal Hypertension (See Chapter 70A, Chapter 70B )
The presence of portal venous pressure greater than 10 mm Hg is often associated with esophageal varices, splenomegaly, and thrombocytopenia (<100,000). Undiagnosed and latent portal hypertension in a cirrhotic patient undergoing liver resection puts the patient at risk of major complications in the postoperative period; such complications include variceal bleeding, endotoxemia, and hepatic decompensation. In a prospective study in class A cirrhotic patients, it was shown that the hepatic venous pressure (HVP) gradient, a surrogate measurement of portal venous pressure, was the only predictor of hepatic decompensation following hepatic resection (Bruix et al, 1996). Thus liver resection in cirrhotic patients with esophageal varices is contraindicated; however, several authors have shown that portal hypertension is not an absolute contraindication to surgical resection in selected patients with preserved liver function (Capussotti et al, 2006; Ishizawa et al, 2008). These authors advocate an aggressive preoperative prophylactic treatment for portal hypertension, such as ligation of esophageal varices.
The negative effect of thrombocytopenia on liver regeneration can be related to the presence of portal hypertension or its direct impact on liver regeneration (Lesurtel & Belghiti, 2008). The development of venous collaterals in patients with elevated portal pressure can reduce the hepatic portal flow and thereby impair the regenerative capacity of the remnant parenchyma after liver resection, therefore we contraindicate major liver resection in cirrhotic patients with venous collaterals.
Liver Atrophy and Underlying Disease
Liver atrophy is a major prognostic factor that should be considered before any liver resection, even those in patients with preserved liver function (see Chapters 5 and 50B). In our practice, we do not consider major liver resection in HCC patients with liver atrophy. Similarly, the underlying etiology of liver cirrhosis should be considered before liver resection, and it should be noted that the extent of fibrosis is more severe in patients with hepatitis C than in patients with hepatitis B (Roayaie et al, 2000). Our tendency is to consider a major liver resection more favorably in patients with hepatitis B than in patients with some other etiology of CLD, even if the remnant liver volume is similar.
Among patients with CLD, the postoperative risk is higher in those with cirrhosis (F4) than in those with extensive fibrosis (F3) (Farges et al, 1999). This suggests that preoperative liver biopsy should be considered in all candidates for major liver resection for histologic fibrosis grading. In patients with F3 or F4 fibrosis requiring major hepatectomy, preoperative portal vein embolization should be performed in an effort to decrease morbidity.
Volume of the Future Liver Remnant
Measurement of the volume of the remnant liver after planned resection using CT volumetry has been shown to be helpful in selecting patients for major hepatic resection (see Chapter 2, Chapter 93A, Chapter 93B ). A small liver remnant volume is associated with worse postoperative liver function and a higher complication rate after extended hepatectomy (Ribero et al, 2007). The safety limit for the remnant liver volume in patients with normal liver is approximately 30% of the total nontumorous or functional liver volume, but this is much higher in patients with CLD. The safety limit in terms of remnant liver volume in patients with CLD has not been clearly documented, and it varies depending on the severity of cirrhosis, which influences the regeneration capacity of the diseased parenchyma. But in general a remnant liver volume of 40% to 50% of the total liver volume should be left before considering major liver resection (Azoulay et al, 2000; Lee & Hwang, 2005; Ribero et al, 2007).
Role of Preoperative Portal Vein Embolization
For patients who require major or extended hepatectomy but have inadequate remnant liver volume, preoperative portal vein embolization (PVE) can be used to induce hypertrophy of the remnant liver (Makuuchi et al, 1990; see Chapter 93A, Chapter 93B ). A general concern is that preoperative PVE may be less effective in the cirrhotic liver, compared with normal liver, because of its impaired regenerative capacity (Abdalla et al, 2002); however, recent studies have demonstrated that preoperative PVE is effective in inducing hypertrophy of remnant liver and decreasing postoperative complications in patients with cirrhotic livers undergoing hepatic resection for HCC. Our center also used PVE in selected F3 and F4 cirrhotic patients undergoing major hepatectomy. The procedure was well tolerated, but the degree of hypertrophy varied according to the degree of fibrosis and the severity of cirrhosis (Farges et al, 1999).
Absence of hypertrophy of the FLR should be considered an absolute contraindication for hepatic resection (Belghiti & Ogata, 2005; Sato et al, 2000); however, we showed that preoperative sequential transarterial chemoembolization (TACE) and PVE increased the hypertrophy of the FLR. In addition, we found a clear relationship between the rate of hypertrophy and postoperative risk after major hepatectomy in cirrhotic patients. These results were recently confirmed by a similar study, which showed that sequential TACE and PVE increase the hypertrophy rate of the remnant liver and are associated with improved overall and recurrence-free survival in patients with HCC, compared with those who have PVE alone (Yoo et al, 2010). After right hepatectomy for HCC, all postoperative deaths and most severe complications were experienced in F4 patients with less than a 10% increase of FLR. Based on these observations, we propose that a minimum increase of 10% of the FLR in F3 or F4 patients is essential to safely perform a right hepatectomy.
Operative Techniques
The general techniques of liver resection are described in Chapters 90A and 90B. This chapter focuses mainly on operative techniques specifically related to the cirrhotic liver.
Skin Incision
A bilateral subcostal incision is the standard incision for hepatic resection and is adequate for most cases of hepatectomy; an extended right subcostal incision is an acceptable alternative. The approach to large tumors located in the upper segment of the right liver or in its posterior surface (segment VII) can be facilitated by an upward midline extension or by a J-incision (Sato et al, 2000). Although the diaphragm incision increases the rate of pleural effusion, it facilitates parenchymal transection by making the transection plane perpendicular to the wound. A study by the Hong Kong group (Xia et al, 2003) showed that a thoracoabdominal approach for right-sided hepatectomy does not increase operative morbidity, compared with an abdominal approach alone, and may help reduce blood loss and transfusion requirements. For cirrhotic patients with HCC in the superior segments of the liver located beneath the right diaphragm, an exclusive transthoracic approach has been described, but this approach allows only limited resection with a high risk of positive margin (Pocard et al, 2002).