Fig. 8.1
PET/CT confirmation of CT findings. CT and PET/CT scan in a 59-year-old female with an adenocarcinoma in the head of the pancreas with suspicious hypodensities in the right lobe of the liver. PET/CT demonstrated these lesions to be hypermetabolic. Arrows point to liver metastases (Reproduced with permission from Farma et al. [52])
8.4 Treatment
8.4.1 Resection of Liver Metastases
Synchronous liver metastasis from pancreatic cancer, regardless of the number or size, has long been considered a marker of systemic disease and poor tumor biology. In turn, synchronous liver metastasis is generally considered a strong absolute contraindication to resection of the pancreatic primary tumor. In a number of small series, patients who underwent an aggressive simultaneous resection of both the primary pancreatic tumor and the metastatic hepatic lesions were noted to do poorly with very limited survival. Takada et al. reported on 11 patients with pancreatic adenocarcinoma who had synchronous liver metastasis and underwent concomitant pancreaticoduodenectomy and partial liver resection [15]. The authors noted no difference in the overall survival of patients who underwent palliative bypass alone versus patients who underwent resection of both the primary and metastatic pancreatic cancer (6 vs. 4 months, respectively). In fact, all patients who underwent pancreaticoduodenectomy with resection of synchronous liver metastasis died from multiple recurrent liver metastases within 1 year [15]. In a separate study, Gleisner and colleagues reported on a small group of patients with synchronous liver metastasis who underwent surgical resection [16]. In this series, the extent of hepatic disease in the liver was minimal (median tumor size 0.6 cm, 91 % patients with 1 liver metastasis), yet simultaneous resection of the pancreas primary adenocarcinoma and the liver metastasis yield a median survival of only 5.9 month, which was no different than the control group who underwent palliative bypass only (Fig. 8.2). In fact, simultaneous resection of the pancreas primary and the liver metastasis resulted in increased morbidity, length of stay, and mortality [16]. In a follow-up study from the group, de Jong et al. reported similar morbidity and mortality between simultaneous and stage hepatic resections for liver metastasis from periampullary malignancies [17]. In another study by Shrikhande et al. of 11 patients who underwent combined pancreatic and liver resection for metastatic pancreatic cancer, the authors reported a median survival of 11.4 months [18]. Klein et al. reported on 22 patients with pancreatic adenocarcinoma metastatic to the liver who underwent simultaneous liver resection and pancreatectomy. In this small cohort of patients, the postoperative morbidity and mortality were no higher among patients who underwent pancreatectomy alone; however, the overall survival among patients who underwent resection in the setting of synchronous liver metastasis was very poor [19]. In contrast, Yamada et al. reported somewhat better results among six patients who underwent simultaneously resection of pancreatic cancer and liver metastasis [20]. Specifically, the authors noted an overall 1-, 3-, and 5-year survival after combined hepatectomy and pancreatic resection of 66.7, 33.3, and 16.7 %. In fact, one patient with liver metastasis was alive at 65.4 months following surgery; however, it is important to note that this patient had metastasis from a pancreatic cystadenocarcinoma, which may have a different biologic behavior compared with pancreatic ductal adenocarcinoma [20].
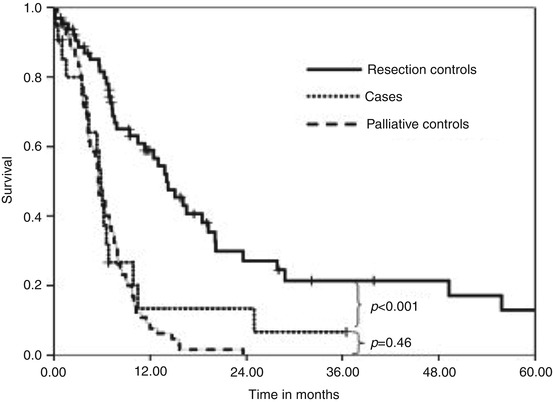

Fig. 8.2
Results of matched survival analysis. Patients (n = 22) who underwent simultaneous resection for primary adenocarcinoma and for liver metastasis had a similar overall survival (median, 5.9 months) compared with patients (n = 66) who underwent palliative bypass alone (5.6 months; p = 0.46), but they had a significantly shorter survival compared with patients who had no liver metastasis and, thus, underwent resection of the primary tumor alone (median, 14.1 months; p < 0.001) (Reproduced with permission from Gleisner et al. [16])
In aggregate, resection of synchronous liver metastasis from pancreatic adenocarcinoma should be avoided. While the level of data is poor, being based on small retrospective cohort studies, findings are generally consistent. Patients offered surgery for pancreatic adenocarcinoma and synchronous liver metastasis do very poorly. Overall survival is short and recurrence is nearly universal. As such, patients with pancreas adenocarcinoma who have liver metastasis diagnosed preoperatively should not be offered surgery. When unsuspected synchronous liver metastases are discovered at the time of surgery, resection of the primary tumor with concomitant resection of the liver lesions should also be strongly discouraged. The data would suggest that even when the burden of disease in the liver is small that these patients derive little benefit from surgery [18]. Rather patients should undergo a palliative procedure if warranted and then be referred for chemotherapy. Avoiding a bypass procedure (unless indicated) may decrease recovery time and allow earlier initiation of chemotherapy.
The possible role of resection for metachronous pancreatic adenocarcinoma to the liver is more controversial. Whereas most surgeons agree that up-front resection of synchronous disease is virtually never warranted, some clinicians have suggested that resection of well-selected patients with metachronous disease may provide a survival benefit [21]. For example, Dünschede et al. reported on 23 patients with pancreatic liver metastases, 14 of whom had synchronous disease and 9 of whom had metachronous disease [21]. Compared with gemcitabine-based chemotherapy, surgery did not provide a survival benefit for patients with synchronous liver metastasis (8 months vs. 11 months). In contrast, among patients with metachronous liver metastases, surgical resection was associated with a median survival of 31 months compared with only 11 months for those patients treated with chemotherapy alone. Klempnauer et al. have shown a median survival of 8.3 months after curative resection of synchronous PCLM (n = 16) and 5.8 months after resection of metachronous PCLM (n = 7) (Fig. 8.3; Table 8.1) [22]. In a separate study, Adam et al. reported on liver resection for metachronous liver metastases among 40 patients with primary pancreatic exocrine cancer [23]. In this subset of 40 patients with metachronous pancreatic adenocarcinoma liver metastasis, the estimated 5-year survival following liver resection was 20 % – better than anticipated for patients with stage IV disease. Patients with metastases from pancreatic adenocarcinoma who underwent hepatic resection had, however, a lower survival than those with metastases from ampullary primary tumors [23]. In a separate study, Gleisner and colleagues reported a small group of patients with periampullary cancer with synchronous liver metastasis who underwent surgical resection [16]. Patients with nonpancreatic primary cancer (duodenal or ampullary) had longer median survival compared with those who underwent resection of pancreatic cancer liver metastasis [16]. This was confirmed by study from de Jong et al. who showed median survival following resection of liver metastases from intestinal-type tumors (n = 15) was 23 months compared with 13 months for patients with pancreaticobiliary tumors (n = 25) (p = 0.05) [17]. In a systematic review of data from 103 patients with liver metastases from pancreatic adenocarcinoma, Michalski and colleagues reported that resection of metachronous lesions was safe and associated with a median survival of 5.8–11.4 months [24].
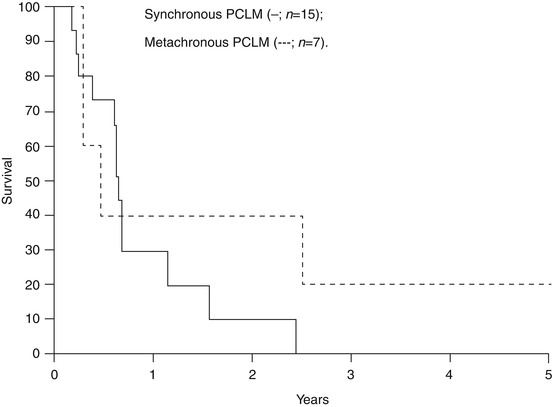

Fig. 8.3
Survival after synchronous resection of the pancreatic primary tumor and the liver metastasis (—; n = 15) and after metachronous resection of liver metastases of pancreatic cancer (– – – –; n = 7) (Reproduced with permission from Klempnauer et al. [27])
Table 8.1
Summary of studies on resection of PCLM
Author (year) | # patients with pancreatic cancer liver metastasis | Median survival | 1- and 5-year actuarial survival |
|---|---|---|---|
Klempnauer et al. (1996) [22] | 15 synchronous | 8.3 months | 41 and 0 % |
Takada et al. (1997) [15] | 11 synchronous | 4 months | 0 and 0 % |
Yamada et al. (2006) [20] | 6 synchronous | n/a | 67 and 17 % |
Gleisner et al. (2007) [16] | 17 synchronous | 5.9 months | 13 and 0 % |
Shrikhande et al. (2007) [18] | 11 synchronous | 11.4 months | 42 and 0 % |
Klein et al. (2012) [19] | 22 synchronous | 7.6 months | 30 and 0 % |
Dünschede et al. (2010) [21] | 9 metachronous | 31 months | n/a |
Adam et al. (2006) [23] | 40 metachronous | 20 months | n/a and 25 % |
While patient selection clearly plays a very important part in explaining the survival benefit associated with surgery, these data suggest that liver resection may be warranted in a select subset of patients with metachronous disease. Selection of patients for resection of metachronous liver metastasis from pancreatic cancer needs to be very stringent. In addition, candidates for surgery should have a good performance status, adequate future liver remnant, and have disease that is amenable to a complete R0 resection. Typically, only patients who have been treated and who have responded to systemic chemotherapy and those patients with a long disease-free interval should even be considered for possible surgical resection [25]. The duration of the disease-free period and/or the period of time that the patient should be treated with chemotherapy remains ill defined. In general, it is the authors’ opinion that patients should have stable disease, a partial response, or have no evidence of progressive disease for at least 6–12 months prior to surgical resection of liver metastases.
8.4.2 Other Locoregional Treatment Options
In addition to surgery, other locoregional options are sometimes considered in treating patients with hepatic metastasis from pancreatic adenocarcinoma. Ablation, either radiofrequency or microwave, is a potential locoregional option. The same principles that apply to resection of pancreatic liver metastasis also apply to the local thermal destruction of such liver disease. Local destruction of pancreatic liver metastasis is therefore utilized very infrequently.
Intra-arterial therapy (IAT) is another locoregional approach that is sometimes utilized to treat malignant liver disease. While IAT is utilized for certain types of metastatic disease such as neuroendocrine and colorectal liver metastasis, its role in treating pancreatic adenocarcinoma liver metastasis is much more limited. In one small study, Azizi et al. reported on 32 patients with pancreatic adenocarcinoma liver metastasis who were treated with trans-arterial chemoembolization (TACE) at 4–8-week intervals [26]. Following TACE, 71.9 and 9.4 % of patients had either stable disease or a partial response, whereas 18.8 % of patients had progressive disease. The 1-, 3-, and 5-year survival from the time of the first TACE was 60, 25, and 11 %, respectively. Furthermore, survival was better among patients with oligonodular disease (<5 lesions) versus multinodular disease (≥5 lesions) (1-year survival: oligonodular, 84 % vs. multinodular, 50 %) [26]. As such, the authors postulated that TACE might control liver metastases from pancreatic cancer in a certain subset of patients. In a separate study, Tajima et al. investigated hepatic arterial infusion chemotherapy with gemcitabine (GEM) plus 5-fluorouracil or oral S-1 in seven patients with metachronous liver metastases from pancreatic cancer. Three of the seven cases showed a partial response, and stable disease was achieved in another three of the seven cases (response rate, 85.7 %). While no life-threatening toxicities arose, six patients (85.7 %) developed catheter-related complications [27].
In addition to the biologic and oncologic issues that make thermal ablation and IAT of generally not applicable for pancreatic hepatic metastasis, the risk of infectious complications can also make this therapeutic approach undesirable. Specifically, following pancreaticoduodenectomy and the creation of a bilioenteric anastomosis, the biliary tree can become colonized with enteric flora. In turn, liver abscess can be much more common after any liver-directed intervention in the presence of a biliary-enteric anastomosis. Elias et al. reported on percutaneous liver RFA among 11 patients with an enterobiliary anastomosis or biliary stenting [28]. The incidence of liver abscess rate was about 40–50 % after RFA [28]. In another study, De Jong and colleagues reviewed liver-directed therapy in 126 patients with pancreatic liver metastases including 42 patients with pancreatic adenocarcinoma hepatic metastases [29]. Following liver-directed therapy (RFA or IAT), the overall morbidity rate was 34 %, and most complications were major (Clavien grade >3) requiring an invasive intervention [29].
Another locoregional option involves the use of radiation therapy. Traditionally, the liver had been considered to be very radiosensitive, and therefore radiation therapy was utilized somewhat sparingly. More recently, however, radiation therapy has been increasingly used to treat liver tumors. Wiley et al. reported on the results of a prospective study of 17 patients with pancreatic ductal adenocarcinoma liver metastasis who underwent a 2-week hepatic artery infusion of 5-fluorouracil and irradiation of the liver with 20 Gy. The authors noted that the combination of 5-fluorouracil infusion and hepatic irradiation may suppress subclinical (microscopic) liver metastases without significant hepatic toxicity [30]. The efficacy of prophylactic low-dose whole irradiation to the liver was, however, marginal. In a separate study, De Jong et al. reported on the use of adjuvant “prophylactic” whole-liver irradiation (5-fluorouracil plus 30 Gy external beam) in 28 patients with primary pancreatic adenocarcinoma with no clinical evidence of liver metastasis. The treatment was well tolerated, and only one patient developed radiation hepatitis and a liver abscess [29]. Low-dose whole-liver radiotherapy can benefit patients with symptomatic liver metastases with few side effects [31]. Bydder et al. treated 28 patients who had symptomatic liver metastases with liver irradiation consisting of 10 Gy in two fractions over 2 days. Individual symptom response rates were 53–66 % at 2 weeks. Partial or complete global symptomatic improvement was noted in 15 patients (54 %) overall. The treatment was well tolerated with two patients (7 %) experiencing grade 3 toxicity (one vomiting and one diarrhea).
More recently, the use of conformal/stereotactic radiation therapy to ablate liver metastases has emerged with the development of 3D imaging. Conformal radiation therapy uses a multi-leaf collimator (3–4 Fields) to deliver higher doses of radiation to the targeted tumor area. With better imaging guidance, many modulated fields (5–100 s fields) can be precisely focused on the tumor using stereotactic body radiation therapy (SBRT). Goodman et al. reported their experience using SBRT in 19 patients with liver metastases from 2004 to 2008. There were three patients with pancreatic cancer liver metastasis. The prescribed radiation dose was escalated from 18 to 30 Gy at 4-Gy increments with a planned maximum dose of 30 Gy. All patients tolerated the single-fraction SBRT well without developing dose-limiting toxicity. After a median of 17 months follow-up (range, 2–55 months), the cumulative risk of local failure at 12 months was 23 %. Median survival was 28.6 months, and the 2-year actuarial overall survival was 50.4 % [32]. Ideal candidates for SBRT should have good performance status (ECOG 0–1), adequate liver function, no extrahepatic disease, and uninvolved liver volume of 700 ml or greater [33]. There is no current phase III trial showing survival benefit of SBRT for patients with liver metastasis. However, an international survey of radiation therapy (RT) of liver metastases done by the Liver Cancer Workgroup of the Third International Consensus on Metastases Workshop at the 2010 American Society for Radiation Oncology (ASTRO) meeting showed a 36 % increase in the average annual number of referrals for liver radiation therapy (RT) over the past 5 years. The majority of experience in liver metastases is with SBRT for focal metastases rather than low-dose palliative whole-liver RT for symptom control [34].
8.4.3 Systemic Chemotherapy Options
The overwhelming majority of patients with pancreatic adenocarcinoma liver metastases will not be candidates for surgery. Liver metastasis almost universally signifies additional systemic disease, and, as noted, the results with surgery are largely poor. As such, systemic chemotherapy constitutes the backbone of therapy for patients with liver metastasis and pancreatic adenocarcinoma. Gemcitabine (Gemzar™; 2′,2′-difluorodeoxycytidine) is a pyrimidine antimetabolite and a specific analogue of deoxycytidine. Since 1997, gemcitabine monotherapy has been the standard first-line treatment for patients with metastatic pancreatic ductal adenocarcinoma [35]. While there have been numerous attempts to identify a suitable companion drug for gemcitabine as doublet therapy, many phase 3 trials had failed to establish the superiority of any doublet regimen over monotherapy with gemcitabine alone. Recently, the MPACT (Metastatic Pancreatic Adenocarcinoma Clinical Trial) trial demonstrated in a randomized phase 3 trial that gemcitabine combined with nab-paclitaxel led to an improvement in survival among patients with metastatic pancreatic ductal adenocarcinoma, including patients with liver metastases. Specifically, for patient with pancreatic adenocarcinoma liver metastasis, overall survival and progression-free survival was better in the gemcitabine plus nab-paclitaxel group versus the gemcitabine-only group (Hazard ratio for death 0.69, 95 % CI 0.59–0.81; hazard ratio for progression 0.65, 95 % CI 0.54–0.78) [36]. Gemcitabine plus nab-paclitaxel is now the standard of care for this patient group.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







