Fig. 3.1
Liver metastases from kidney cancer on CT scan (arterial phase)
3.3.3 Magnetic Resonance (MR)
Magnetic resonance imaging is commonly used following CT if there are still issues related to diagnosis or staging of the tumor. In particular it is frequently used to evaluate infiltration of closer organs (e.g., liver involvement in case of right kidney cancer). Liver metastases from RCC are usually hypervascular in gadolinium-enhanced MR imaging such as neuroendocrine tumors, melanoma, and thyroid carcinoma. Liver metastases are hypointense on the T1-weighted image and iso-hyperintense on the T2-weighted image (Figs. 3.2 and 3.3). Morphologic, signal intensity, and contrast enhancement features have been shown to be useful in distinguishing metastatic lesions from common benign lesions such as hemangiomas and cysts.
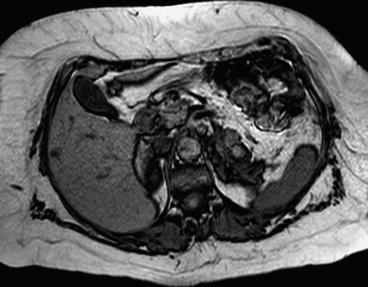
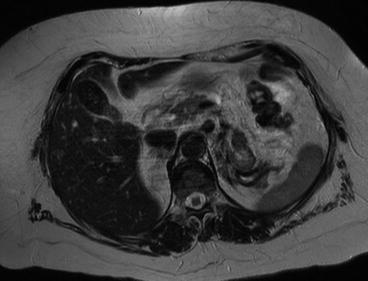

Fig. 3.2
Liver metastases from kidney cancer on RM (T1 weight)

Fig. 3.3
Liver metastases from kidney cancer on RM (T2 weight)
3.3.4 PET Scan
PET scan is not a recommended technique for the diagnosis and follow-up of liver metastases from kidney cancer due to histopathological characteristics of primary tumor. In low-grade kidney cancer, PET is often negative, and evaluation of primary cancer is therefore difficult. Indeed significant uptakes of FDG are normal in kidneys because of the inability of the nephron to reabsorb filtered FDG in the convoluted tubules. That is why PET scan is often falsely positive in kidney cancer, and it is not a recommended technique in diagnosis and follow-up of liver metastases from kidney cancer as well [18].
3.3.5 Staging
The TNM staging system for kidney cancer defines local extension of the primary tumor (T), involvement of regional lymph nodes (N), and presence of distant metastases (M) (Tables 3.1, 3.2 and 3.3). The incidence of liver metastases is associated with local extension of primary tumor (T) (Table 3.1). Synchronous liver metastases are rare in T1a tumors (<4 cm).
Table 3.1
TNM for RCC
T0 | No evidence of primary tumor | – | – |
T1 | Tumor ≤7 cm in greatest dimension, limited to the kidney | <1 % | 1–2 % |
T1a | Tumor ≤4 cm in greatest dimension, limited to the kidney | <0.5 % | 1 % |
T1b | Tumor >4 cm but not >7 cm in greatest dimension, limited to the kidney | 1 % | 1–2 % |
T2 | Tumor >7 cm in greatest dimension, limited to the kidney | 2–6 % | 4–12 % |
T2a | Tumor >7 cm but ≤10 cm in greatest dimension, limited to the kidney | 2–3 % | 4–6 % |
T2b | Tumor >10 cm, limited to the kidney | 4–5 % | 7–12 % |
T3 | Tumor extends into major veins or perinephric tissues but not into the ipsilateral adrenal gland and not beyond Gerota fascia | 6–14 % | 12–27 % |
T3a | Tumor grossly extends into the renal vein or its segmental (muscle containing) branches, or tumor invades perirenal and/or renal sinus fat but not beyond Gerota fascia | 6–8 % | 12–16 % |
T3b | Tumor grossly extends into the vena cava below the diaphragm | 8–10 % | 18–23 % |
T3c | Tumor grossly extends into the vena cava above the diaphragm or invades the wall of the vena cava | 12–14 % | 20–27 % |
T4 | Tumor invades beyond Gerota fascia (including contiguous extension into the ipsilateral adrenal gland) | 12–20 % | >30 % |
Table 3.2
TNM for RCC
N | NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastases | |
N1 | Metastases in a single regional lymph node | |
N2 | Metastases in more than one regional lymph node | |
M | MX | Distant metastases cannot be assessed |
M0 | No distant metastases | |
M1 | Distant metastases |
Table 3.3
RCC stage
Stage | T | N | M |
|---|---|---|---|
I | T1 | N0 | M0 |
II | T2 | N0 | M0 |
III | T1 or T2 | N1 | M0 |
T3 | N0 or N1 | M0 | |
IV | T4 | Any N | M0 |
Any T | Any N | M1 |
In a recent study Kunkle et al. [19] analyzed the incidence of synchronous metastases in 360 patients with kidney cancer and demonstrated that tumors associated with synchronous metastases were significantly larger than those in patients without secondary lesions. In this study there were no patients with tumors <2 cm who presented with confirmed metastatic disease, and less than 5 % of all synchronous metastases occurred in tumors <3.0 cm. In the series of Thompson et al., only 1 patient of 781 with kidney tumor <3 cm had synchronous liver metastases [20].
The incidence of metachronous liver metastases after renal resection is associated with the size of primary tumor. The overall incidence of metastases is, respectively, 3, 13, and 29 % for pT1a, pT1b, and pT2 after 5 years from primary tumor resection, and, when metastases are present, liver involvement is observed in 20.3 % of cases (Table 3.1) [20].
When tumor grossly extends into the renal vein or into its segmental branches, invades perirenal and/or renal sinus fat without passing Gerota fascia (pT3a), extends into the vena cava below or above the diaphragm (pT3b, pT3c), or invades beyond Gerota fascia (pT4), the incidence of synchronous and metachronous metastases is higher (30–70 %), and the incidence of liver metastases is 25–50 %.
3.4 Treatment
Options for treatment of liver metastases from renal cancer include surgery, ablative therapies, and chemotherapy. Indications vary depending on the stage of the disease, presence of extrahepatic metastases, and general status of patients and are evaluated on a case-by-case analysis in a multidisciplinary approach with a synergy of surgeons, oncologists, radiologists, and radiotherapists.
3.4.1 Surgery
Liver metastases develop in 30–40 % of patients with metastatic kidney cancer [21] and in most cases coexist with multiple extrahepatic metastases. Prognosis of these patients is generally poor, and without treatment most patients die within 4 months after diagnosis with only 10 % 1-year overall survival. In the series of Suppiah et al., the median survival of 186 untreated patients with hepatic metastases from renal cell carcinoma was 7.8 months [22]. However, in 2–4 % of patients, metastases are limited to the liver, and patients can therefore be candidates to liver resection. Thanks to the development of surgical technique, liver resection is presently a procedure with low mortality (0–2 %) and acceptable morbidity (20–30 %) [23] and represents the only potentially curative treatment for patients with metastases from renal cell carcinoma, offering the chance for long-term survival: indeed renal carcinomas and their metastases rarely respond to traditional systemic chemotherapy, radiotherapy, or hormone modulation therapy.
Synchronous Liver Metastases
Synchronous liver metastases represent about 10 % of liver metastases from kidney cancer. The subset of patients with potentially surgically resectable primary RCC and resectable liver metastases (2–5 %) are candidates to nephrectomy and liver resection. Candidates include patients who initially present with primary RCC and both solitary or multiple resectable liver metastases. Primary tumor and liver metastases may be resected during the same operation or through staged procedures. The most frequent surgical strategy is the second one, while simultaneous nephrectomy and hepatic resection are uncommonly performed during a combined procedure.
Sakaguchi et al. in 1992 demonstrated the feasibility of simultaneous liver resection and nephrectomy for a large RCC with caval thrombus [24]. The main problem to deal with during combined procedure is that liver resection and nephrectomy need conflicting hemodynamic techniques: during nephrectomy, a normovolemic or hypervolemic state is preferred to supply the contralateral kidney with adequate volume, therefore maximizing renal plasma flow to the remnant kidney. By contrast, relative hypovolemia is desired during hepatic resection (low central venous pressure technique) to limit blood loss during parenchymal transection phase of the operation. Anyway, in patients needing caval thrombectomy, hypoperfusion is especially dangerous because of an increased risk of thrombosis [25].
In the most series, the most frequent indication to combined renal and hepatic surgery is instead locally advanced renal cell carcinoma. Outcome of combined procedures is reported only in few limited series from literature [26, 27]; therefore, indications, perioperative management, and postoperative outcomes for simultaneous liver and kidney resection remain poorly defined. In a recent single-center study, Yezhelyev et al. [25] analyzed outcome of 20 patients who underwent synchronous hepatic and renal resection. To optimize perioperative fluid management, hepatectomy was performed before nephrectomy whenever possible. This strategy allowed maintenance of a low central venous pressure (CVP) technique during hepatic parenchymal transection, followed by fluid repletion before and during nephrectomy. Low central venous pressure was maintained as long as adequate urine output of 25 mL/h and systolic blood pressure of 90 mmHg were preserved during transection of liver parenchyma. At the conclusion of the liver resection, crystalloids were administered to achieve a relative hypervolemic state for optimal kidney resection. In this series, 50 % of patients developed major postoperative complications, and postoperative mortality was 5 %. Pulmonary complications were among the most common in the postoperative period (30 %), followed by intra-abdominal (15 %) and surgical site infections (15 %).
Nephrectomy associated with liver resection can therefore be offered to carefully selected patients with synchronous neoplasms of the liver and kidney in centers with high volume of liver and kidney resection. Because of the low number of study about simultaneous hepatic and renal resection, indications of this procedure are still not codificated.
In patients with unresectable synchronous liver metastases, cytoreductive nephrectomy before systemic therapy is recommended. Many randomized clinical studies show that cytoreductive surgery in association with systemic therapy provides a significant survival benefit compared with systemic therapy alone for patients with metastatic RCC [28]. The European Organization for Research and Treatment of Cancer reported a trial where patients with metastatic RCC were randomized to treatment with INF-alpha or INF-alpha plus radical nephrectomy. In the group submitted to radical nephrectomy, the overall survival was 17 months compared to a survival of 7 months reported for patients without nephrectomy [29]. Cytoreductive surgery of primary tumor has been for decades the gold standard treatment for metastatic RCC because spontaneous regression of metastases was expected upon primary cancer removal. Furthermore, surgical resection of large symptomatic cancer may have a significant palliative effect even in terms of quality of life.
Surgical option includes radical nephrectomy and nephron-sparing surgery. A radical nephrectomy requires proximal ligation of the renal artery and vein and removal of the kidney with Gerota fascia, ipsilateral adrenal gland, and regional lymph nodes. Radical nephrectomy is the preferred treatment for tumors extending into the inferior vena cava. Nephron-sparing surgery was originally indicated only if a radical nephrectomy would have rendered patient functionally anephric, necessitating dialysis. It was also indicated in patients with hereditary forms of RCC who may have multiple tumors and in patients with a unilateral tumor and functioning contralateral kidney affected by renal artery stenosis, hydronephrosis, chronic pyelonephritis, reflux, kidney stones, or systemic conditions such as hypertension or diabetes [30]. However, nephron-sparing surgery has been used increasingly in patients with a normal contralateral kidney, with long-term outcomes comparable to radical nephrectomy [31]. The additional benefits of nephrectomy and nephron-sparing surgery include improved performance status, elimination of paraneoplastic syndromes, and eradication of the source of new metastases. Many series in literature showed that survival in patients with metastatic kidney cancer underwent nephron-sparing surgery of the primary tumor and patients underwent radical nephrectomy was comparable [32].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







