Author
Year
Patients (n)
LMs (n)
Diagnosis tool
Treatment
Hormone status
Time since PC diagnosis (mon)
Survival after LM diagnosis (mon)
HSPC
CRPC
HSPC
CRPC
HSPC
CRPC
Wang
2013
27
NA
US/CT/MRI/B
ADT/CT/RT
8
19
NA
14
38
6
Pouessel
2007
28
NA
US/CT/B
HT/CT
1
27
NA
NA
NA
6
Kelly
2012
59
NA
NA
D, P with either B or placebo
59
NA
NA
NA
NA
Pezaro
2014
71
NA
CT
NA
71
55.2
19.2
NA
NA
Shakir
2008
1
M
CT, A
SC
1
NA
NA
Marech
2013
1
M
CT
AA and P/ZA/Ca
1
51
NA
Batty
2013
1
NA
CT
Ca/Cy and P/AA/Mi
1
20
6
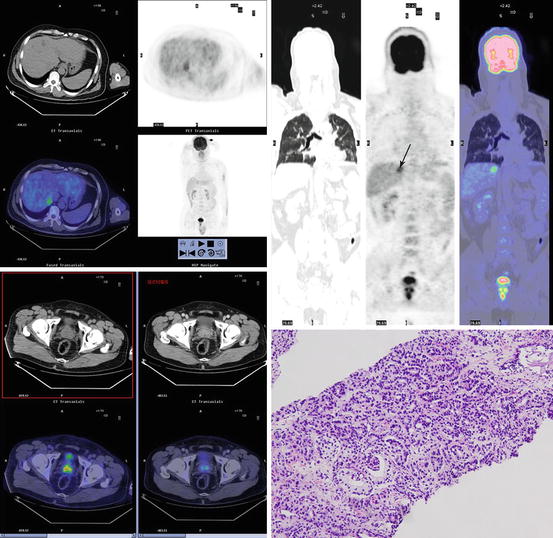
Fig. 15.1
F18 fluorodeoxyglucose positron emission tomography/computed tomography images in a prostate cancer patient with solitary liver metastasis. Axial positron emission tomography and fusion images show increased activity in prostate (down left) and solitary hepatic metastases (upper left). Maximum intensity projection image (upper right) also shows solitary liver metastasis and prostate cancer. Focally increased activity (arrow) is seen in liver. The prostate biopsy showed the low differentiated prostate adenocarcinoma (down right)
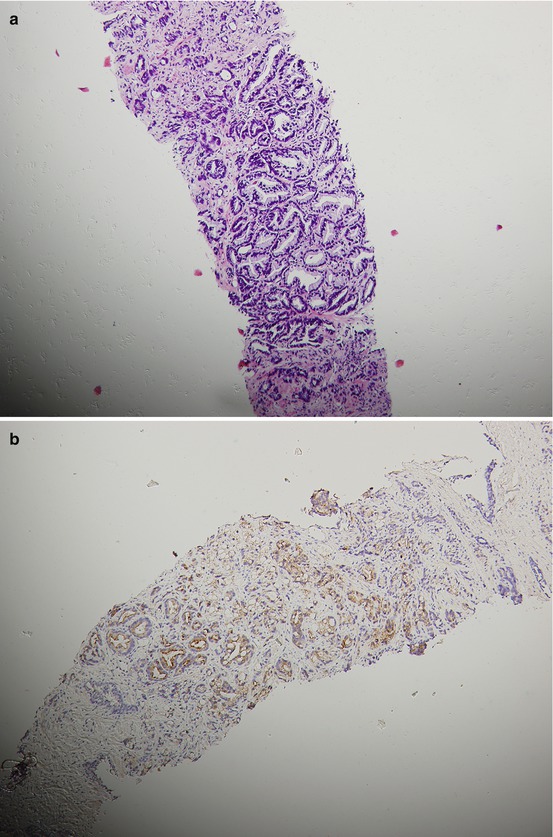
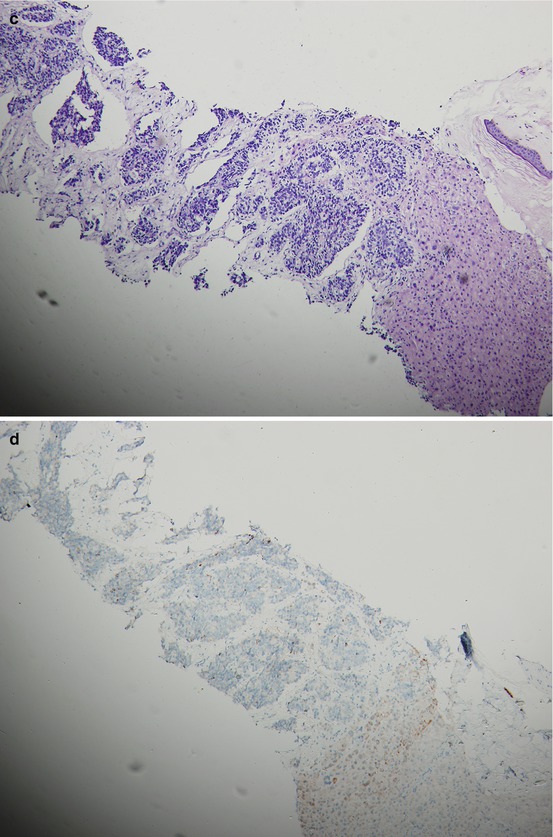
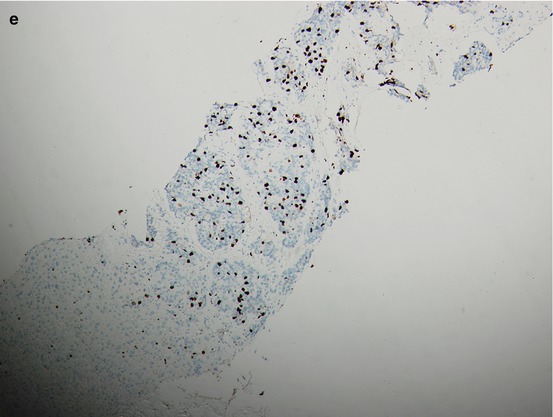
Fig. 15.2
A patient’s tumor tissue at initial pretreatment prostate biopsy (a. b.) and liver biopsy (c–e) at liver metastasis for CRPC with hematoxylin-and-eosin-stained material showing histologic changes from initial biopsy (a) to liver biopsy at CRPC with immunohistochemical staining for P504S and Ki67 on liver biopsy at CRPC (d, e.) and P504S on initial biopsy (b)
15.3 The Prognosis Difference Between Liver Metastases and Non-liver Metastases
CRPC patients with LM usually represent a poor prognosis. Armstrong et al. [5] reported that 640 CRPC patients had received chemotherapy; in their multivariable analysis, LM was associated with post-progression survival (hazard ratio (HR) = 1.48, 95 % confidence interval (CI) 0.94–2.30, P = 0.089). Kelly et al. [3] announced a set of data in ASCO Annual Meeting in 2012, data from 1,050 men treated with docetaxel (D) and prednisone (P) with either bevacizumab (B) or placebo. Patients were chemotherapy naïve and had evidence of progressive mCRPC despite castrate testosterone levels and antiandrogen withdrawal, ECOG performance status ≤2, and adequate bone marrow, hepatic, and renal functions. The median overall survival (OS) time in LM patients was 14.4 compared to 22.6 months, with a HR of 1.4. The HR for treatment effect (DP+B vs. DP) for LM was not statistically significant for either group. Compared to patients without LM, mCRPC patients with LM have a poor OS despite having similar progression-free survival (PFS) and objective biochemical response to docetaxel-based therapy. In these cases, CRPC with LM was reported to be associated with poor prognosis.
However, Pezaro et al. [6] reported that CRPC patients with visceral metastases (VM) may not represent a more aggressive disease phenotype. The association with worse outcome may instead relate to the increased prevalence of VM as overall disease burden increases. Their study also showed that VM involved the liver (20 %) in 359 CRPC patients. Patient outcome differed based on the degree of bone involvement at detection of VM, with a median survival of 18.2 months in men with no evident bone disease (n = 12; interquartile range [IQR]: 24.9; P = 0.001 vs. more than six lesions), 8.1 months in those with moderate bone involvement (fewer than six lesions; n = 18; IQR: 25.6; P = 0.049 vs. six or more lesions), and 6.1 months in those with more extensive bone involvement (six or more lesions; n = 84; IQR: 8.9). Wang et al. [4] reported that LM does not necessarily predict a worse outcome when seen as synchronous LM at the diagnosis of prostate cancer; when seen as a site of progressive CRPC, prognosis was worse.
15.4 Prognostic Factors in Prostate Cancer with Liver Metastasis
Prognostic factors mainly provide the clinician with a more sophisticated means of assessing the potential benefit of treatment. Firstly, these allow a more cogent discussion between doctor and patient with respect to the risks and benefits of therapy. In addition, it may delineate those patients who make up a high-risk group and who may be served well by further aggressive therapy. Most important, these can be used to stratify patients in clinical trials. It should be stressed that prognostic factors are meant not to exclude individuals from given therapy that represents a potentially curative option, but rather to assess the risk of progression and to perhaps select those who should receive additional treatment.
15.4.1 Hormone Status
Wang et al.’s [4] survival analysis showed that hormone status (hormone refractory or hormone sensitive) is a clinically relevant predictor of poor overall survival in prostate cancer with LM after diagnosis of LM and has demonstrated to be statistically significant (P = 0.003).
Wang et al. also reported that in the hormone-sensitive group, the median OS after LM diagnosis was 38 months and half of the patients were still alive at the latest follow-up [4]. This compares with published median survivals between 28 and 52 months for large series employing androgen deprivation for treatment of patients with minimal or extensive metastatic prostate cancer [7]. While in the hormone refractory group, the median OS after LM diagnosis was only 6 months. Pouessel et al. [2] reported the median OS was 6 (1–27) months, confirming the results of Wang et al. [4]. Thus, prostate cancer with LM does not mean poor outcome, it also needs to take into account other factors, including hormonal status.
15.4.2 Neuroendocrine Differentiation
The neuroendocrine pattern is in agreement with the concept of progression from normal prostate to adenocarcinoma and finally to small-cell carcinoma during prostatic tumorigenesis [8]. Neuroendocrine differentiation (NED) in prostate cancer appears to be a poor prognostic factor [8]. In GETUG P01 trial [9], 55 mCRPC patients present with at least one of the following: those with VM (lung, liver, or lymph node involvement) except bone metastases or any elevated neuroendocrine serum marker (chromogranin (CgA) or neuron-specific enolase (NSE)) of ≥1.5-fold normal value, who received the association of etoposide and carboplatin with a median of four treatment cycles, and those with VM with or without neuroendocrine serum markers have an OS 8.9 months, 95 % CI (5.9–9.6), and 11.7 months, 95 % CI (8.7–18.4), respectively. Wang et al.’s study [4] also confirmed the results of GETUG P01 trial. In their study, the NSE level at LM ≥12.5 ng/ml, the median survival was 4 months, 95 % CI (1.228, 6.772); NSE level at LM <12.5 ng/ml, the median survival was 9.6 months, 95 % CI (not evaluable, 19.802), P = 0.042.
NED in prostate cancer is uncommon and highly aggressive with a tendency to systemically metastasize and thus has a poor prognosis, possibly related to the increasing degree of dedifferentiation, resistance to hormonal therapy, and growth stimulation of the tumor by neurosecretory products [10]. Pouessel et al.’s [2] study showed that LM was associated with the NED. NED is known to occur in prostate cancer where it associates with poorly differentiated disease with VM and/or secretion of neuroendocrine serum tumor markers (NSE and CgA) [11]. Wang et al.’s [4] univariable analysis showed that in the hormone-refractory group, high concentration of serum NSE is one of the factors that predict poor survival after LM. In their study, the concentrations of NSE were available after the diagnosis of liver metastases in 14/27 patients. Eight of 14 patients received chemotherapy. During the course of chemotherapy, of the seven patients followed up serially, NSE was raised in all two patients who had normal NSE prior to chemotherapy. Out of five patients with elevated NSE levels, four showed a fall in NSE. In two of the latter, it became normal and all the four patients with a decrease of NSE during the course of chemotherapy showed radiologically assessed partial response (n = 3) or stable disease (n = 1).
Pouessel et al. [2] reported 28 patients with metastatic prostate cancer with LM. Serum measurement of neuroendocrine markers showed high level of CgA and NSE in 84 and 44 % of patients, respectively. For the six patients who had liver biopsies and two were neuroendocrine carcinomas, one of which was poorly differentiated, with a small-cell carcinoma pattern. For immunohistochemistry (IHC), all adenocarcinomas were positive for prostate-specific antigen (PSA), whereas none of the neuroendocrine carcinomas expressed PSA. Conversely, neuroendocrine tumors expressed specific markers such as synaptophysin (Syn), CgA, or neural cell adhesion molecule (N-CAM). Notably, CgA was detected in only one case of neuroendocrine tumor, and thyroid transcription factor 1 (TTF1) was positive in the poorly differentiated neuroendocrine component.
In summary, we may consider that NED is correlated closely with poor prognosis of prostate cancer with LM.
15.4.3 Previous Chemotherapy
Wang et al. [4] also reported that another important prognostic factor for survival is the previous chemotherapy. In their study, five patients with previous chemotherapy, the median survival time was 2 months, 95 % CI (not evaluable, 4.147); 14 patients without previous chemotherapy, the median survival time was 9.6 months, 95 % CI (2.266, 16.934), P < 0.001. But they found that in the previous chemotherapy, a significant prognostic factor does not mean that chemotherapy itself makes survival worse. The possible reason is that those with previous chemotherapy have no or few effective options for chemotherapy left after LM occurred.
15.5 Diagnosis
A variety of modalities, including clinical manifestations, laboratory tests, tumor markers, radiologic examinations, and invasive techniques, are relevant in diagnosing patients with prostate cancer with LM.
15.5.1 Clinical Manifestations
Prostate cancer with LM has been reported to be usually a late manifestation of systemic disease and most often occurs in patients after extensive therapy with hormone and/or chemotherapy [2]. Prostate cancer with LM may present asymptomatically during a metastatic screen or may present with specific symptoms. The most common specific symptoms were as follows: upper abdominal pain, jaundice, and distention of abdomen. Wang et al. [4] reported that 40.74 % had symptoms specific to the liver during the onset of LM, while 59.26 % did not have symptoms specific to the liver. In severe cases with rapidly growing tumors, patients can present with fulminant hepatic failure (FHF) and subsequent multisystem organ failure as a result of homogenous infiltration of the liver by metastatic disease. Shakir et al. [12] presented a case of metastatic prostate adenocarcinoma in which a 68-year-old man presented with FHF. An abdominal CT scan revealed hepatomegaly without focal lesions, and Doppler ultrasound showed hepatomegaly and hepatic steatosis. The patient’s condition rapidly declined, with multiorgan system failure necessitating emergent ICU interventions. Autopsy revealed an enlarged liver weighing 3,478 g, with the biopsy showing intravascular and intraparenchymal widespread metastatic prostatic adenocarcinoma.
15.5.2 Laboratory Tests
When hepatic involvement occurs with tumors, the degree of hepatic enzyme elevation varies upon presentation but is often found to correlate with the degree of intrasinusoidal hepatic involvement. Wang et al.’s [4] study showed that liver function tests are deranged in 13 patients (48 %) at presentation with ALP and AST being the most commonly elevated enzymes.
15.5.3 Tumor Markers
Pouessel et al.’s [2] study showed that LM is associated with the NED. In their study, 28 patients with metastatic prostate cancer with LM, serum measurement of neuroendocrine markers showed high level of CgA and NSE in 84 and 44 % of patients, respectively. Wang et al.’s study [4] also confirmed the results of Pouessel et al.’s. In their study, the concentrations of NSE were available after the diagnosis of LM in 14 patients (52 %). Cussenot et al. [13] reported CgA appears to be the best marker of NED and a correlation has been found between neuroendocrine serum markers and the incidence of distant metastases. Pezaro et al.’s [6] study also demonstrated a patient with LM developed widespread metastatic liver disease despite very low levels of PSA; the biopsy revealed small-cell carcinoma morphology with negative IHC staining for PSA and androgen receptor (AR). Thus, plasma neuroendocrine markers can be used as predictive markers associated with prostate cancer with LM.
15.5.4 Imaging Modality
Ultrasonography
Conventional transabdominal US is routinely used as the first-line imaging modality for the detection of liver metastases, and it also plays a substantial role in the intra-procedural localization of lesions during biopsy and therapeutic interventions such as thermal ablation, providing real-time imaging with no ionizing radiation to the operator.
The reported sensitivity of US is much lower than that of CT or magnetic resonance imaging(MRI), ranging from 40 to 77 %, and can drop to 20 % for small lesions (<1 cm) or for lesions that are isoechoic compared with the surrounding liver parenchyma [14, 15]. Thus, detection of hepatic lesions with US frequently initiates a request for an imaging study with greater accuracy, such as CT or MRI.
The development of new ultrasound contrast agents and sonographic techniques has considerably improved the US detection of hepatic metastases and the evaluation of response to therapeutic procedures. Contrast-enhanced ultrasound (CEUS) has improved the sensitivity for detecting LM to 80–90 %, which is not statistically different than the diagnostic performance of CT, and is especially useful for LM smaller than 10 mm [14]. CEUS alone was reported sufficient to classify 89.0 % of focal liver lesions as benign or malignant, which served as a one-stop diagnostic test for 80.8 % of patients, reducing the need for CT and MRI scans, and provided savings in terms of radiation exposure, time, and money [16].
Computed Tomography
In clinical practice, CT is the mainstay of oncological imaging because of its excellent availability, good patient tolerance, and reproducibility. As metastatic hepatic lesions are usually hypovascular, the optimal CT strategy is helical scanning during the portal venous phase of enhancement. This technique improves lesion identification by increasing the attenuation of normal liver tissue, and occasionally rim enhancement of a hypoattenuating metastasis can be seen [17].
The advent of multidetector row computed tomography (MDCT) effectively replaced computed tomography during arterial portography for diagnosis of hepatic metastases. MDCT is highly accurate for evaluating staging, especially for the detection of lymph node enlargement and distant metastases. The accuracy of MDCT for overall M staging is 85.6 %, and for identifying LM the sensitivity is 80.0 % [18]. CT is a standard test for the detection of the LM; however, the reported sensitivity for small metastases (<1.5 cm) is low [19]. A recent comparison between total liver-volume perfusion CT (CTP) and four-phase CT revealed that CTP increased the sensitivity for the detection of LM to 89.2 % from 78.4 % (P = 0.046) and specificity to 82.6 % from 78.3 % (P = 0.074), indicating that CTP is a noninvasive, quantitative, and feasible technique [20].
Magnetic Resonance Imaging
MRI is considered to be the optimal diagnostic modality for evaluation of suspected LM, with a reported sensitivity of 80–100% and specificity up to 97 % [21]. The diagnostic performance of MRI for small lesions (<1.0 cm) has been shown to be superior to helical CT [22]. Modern comprehensive liver imaging protocols generally use a variety of sequences to optimize lesion detection and characterization. Diffusion-weighted (DW)-MRI has been found to be sensitive for the detection of small liver lesions (<1 cm) that mimic small intrahepatic vessels; however, as there is an overlap of apparent diffusion coefficient (ADC) values between different types of lesions, the predominant limitation of the DW-echo planar imaging (EPI) sequence is the differentiation between benign and malignant lesions [23]. Contrast-enhanced MRI can demonstrate tissue-specific physiological information, thereby facilitating liver lesion characterization, which may facilitate the accurate diagnostic workup of patients in order to avoid invasive procedures for lesion characterization, such as biopsy. Gadolinium-enhanced T2-weighted images and superparamagnetic iron oxide (SPIO)-enhanced T2-weighted MRI have been reported to have the ability to differentiate primary malignant, metastatic, and benign lesions in the liver [24]. However, with the increasing awareness of the potential risks of contrast material administration in patients with severe renal failure, there is a strong clinical need for methods to diagnose LM without exogenous contrast materials. Nasu et al. [25] retrospectively compared the accuracy of respiratory-triggered DW-EPI in combination with unenhanced T1- and T2-weighted imaging versus SPIO-enhanced imaging and found that there was no significant difference of sensitivity and specificity between the two imaging methods. DW-MRI combined with unenhanced T1- and T2-weighted imaging is a reasonable alternative to contrast-enhanced MRI for the detection of LM [25].
Positron Emission Tomography (PET)
18F-FDG PET and FDG-PET with CT (PET/CT) have the added advantage over MRI and CT of providing not only anatomic but also functional information. The major advantages of PET are the ability to detect extrahepatic disease and to evaluate therapeutic response early. One series found that FDG-PET had a sensitivity and specificity of 85 and 74 %, respectively, for the detection of LM originating from gastric cancer [26]. A recent study demonstrated that the detection rate of liver lesions was significantly lower for PET/CT than for gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MRI (64 % vs. 85 %, respectively; P = 0.002), which is especially relevant in small LM (≤1 cm in diameter) [27].
15.5.5 Invasive Techniques
The tumor biopsy is presently defined by standard clinical and pathologic features of patients and their cancers. When metastasizing to liver from prostate cancer, they tend to form discrete lesions that are easily identified by standard radiographic methods, including MRI and CT. However, when the tumor radiographically blends with the normal organ parenchyma or micrometastases that imaging methods cannot detect, the diagnosis of metastatic disease can be difficult. Thus, extensive workup can ensue until a tissue biopsy is obtained. But the physician must pay special attention to the potential risk of bleeding postprocedure when the organ involved is the liver. In summary, quick recognition of this diagnosis and confirmatory biopsy will usually lead to the best available outcome for the patient.
Clinical manifestations and laboratory findings usually are late manifestations in patients with LM. The ideal radiologic approach to screening for LM from prostate cancer remains to be established. However, a variety of modalities, including imaging modality and biopsy, are relevant in diagnosing patients with prostate cancer with LM. Each imaging modality has its relative benefits in the evaluation of prostate cancer with LM. Helical multidetector CT is widely available and can accurately diagnose larger lesions, whereas MRI has superior performance versus helical CT in the detection and characterization of small liver metastases. US is frequently used as a guiding technology for percutaneous lesion sampling and therapeutic interventions. PET-CT is superior for the detection of extrahepatic disease. CEUS is a noninvasive and promising tool for detecting LM, as well as for guidance and the evaluation of response of therapeutic procedures early. Tissue biopsy is the gold standard for diagnosis.
15.6 Treatment
After the patient has been evaluated for the LM from prostate cancer, a number of therapeutic options are available. These include hormone therapy; systemic chemotherapy; abiraterone; surgical resection; other local ablative procedures, such as cryosurgery and hepatic artery embolization; and regional infusion chemotherapy.
15.6.1 Hormone-Sensitive Prostate Cancer with LM
Clinically, the treatment of metastatic prostate cancer is based on castration and androgen deprivation therapy (ADT). For patients with hormone-sensitive prostate cancer (HSPC) with LM, hormone therapy (by orchiectomy, gonadotropin-releasing agonists, or antagonists with the early addition of antiandrogens) is the main strategy.
Castration Therapy
Luteinizing hormone-releasing hormone (LHRH) agonists have become the standard of care in castration therapy because these agents have the potential of reversibility and enable the use of intermittent ADT, avoid the physical and psychological discomfort associated with orchiectomy, have a lower risk of cardiotoxicity than observed with diethylstilbestrol, and result in equivalent oncologic efficacy [28, 29].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







