Site of metastasis
n
Median survival (mo)
95 % CI
HR
95 % CI
p
LN/skin
158
43.0
33.0–53.0
1
1
–
Bone
404
33.2
31.0–38.9
1.62
1.16–2.24
0.004
Lung
184
22.4
18.0–28.0
2.01
1.41–2.88
0.0001
Liver
123
12.0
9.0–15.0
4.30
2.92–6.34
<0.0001
Multiple
143
9.0
7.0–11.0
4.87
3.35–7.06
<0.0001
Brain
26
3.0
1.0–8.0
15.00
8.17–27.50
<0.0001
2.3 Diagnosis
Conventional imaging modalities yield different accuracies in the diagnosis of BCLM (Fig. 2.1). Although ultrasound (US) has limited screening value and is highly examiner dependent, intraoperative US has become crucial for many surgeons to intraoperatively localize BCLM. Enhanced computed tomography (CT) (Fig. 2.1b) yields a sensitivity, specificity, and accuracy of 70, 85, and 82 %, respectively [15, 16]. However, the accuracy is much lower with lesions <1 cm. Magnetic resonance imaging (MRI) (Fig. 2.1c) has a high sensitivity of 80–92 % and a specificity of 85 %. For lesions around 1 cm, MRI is more accurate than CT [17]. More recently, the integration of functional imaging, i.e., positron emission tomography (PET) with 2-deoxy-2-[F-18]fluoro-D-glucose (FDG), to conventional imaging has increased the diagnostic accuracy. In a recent meta-analysis, FDG-PET/CT has reported to have a pooled sensitivity and specificity of 96 and 95 %, respectively, for the detection of distant metastases from BC [18]. Several international guidelines currently recommend the inclusion of FDG-PET or PET/CT to the conventional radiologic modalities for the staging of BC and for the detection of distant metastases [19]. However, the impact of increased sensitivity of FDG-PET on patient care and outcome has not been demonstrated. The data regarding prediction of treatment response are insufficient to reach any conclusion. Several prospective, adequately powered clinical trials are currently in progress to define the exact role of this modality in the management of patients with BC [20]. Furthermore, some new PET biomarkers for receptor expression, cell proliferation, and angiogenesis have been already introduced, the definitive role of which has to be verified through prospective trials for the purpose of treatment monitoring [21].
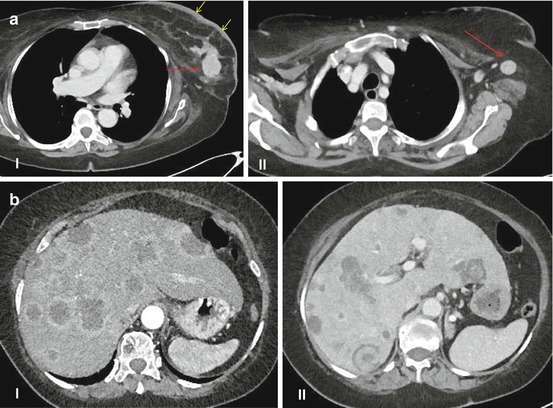
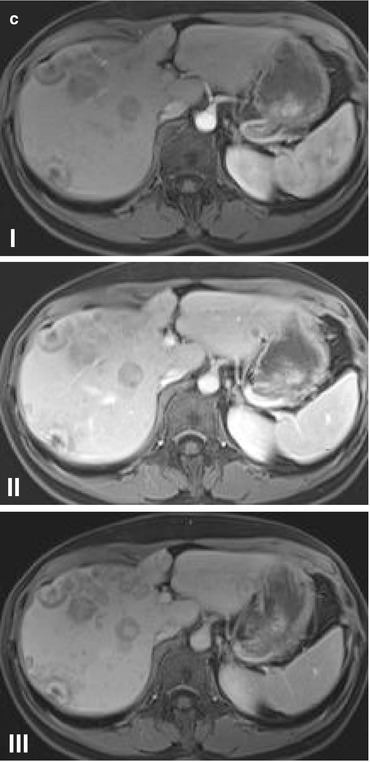


Fig. 2.1
(a) Computed tomography scan of the primary breast cancer (a–I, red arrow) and lymph node metastasis (a–II, red arrow). Note the thickening of the cutis (a–I, yellow arrows) suggesting an inflammatory carcinoma of the breast. (b) Dynamic contrast-enhanced computed tomography of liver metastases of breast cancer depicting arterial (b–I) and portal venous (b–II) phases. (c) Dynamic contrast-enhanced magnetic resonance imaging (MRI) of liver metastases of breast cancer depicting arterial (c–I), portal venous (c–II), and late (c–III) phases (Courtesy of Prof. Dr. Lars Grenacher, Department of Diagnostic and Interventional Radiology, Ruprecht-Karls University of Heidelberg, Germany)
2.4 Treatment
Stage IV BC is almost always incurable. Despite remarkable evolution in the systemic therapy of BC, including immunologic agents (trastuzumab), hormonal therapy (selective estrogen receptor modulators and aromatase inhibitors), and more effective chemotherapeutic protocols, the prognosis of the patients with stage IV BC is still dismal; the median survival of patients with hepatic metastases ranges from 1 to 25 months [22]. In shadow of this poor prognosis, the consideration of stage IV BC as a systemic disease, and lack of an internationally accepted consensus on therapy of metastatic BC, palliative less aggressive treatments with low toxicity profiles have been generally preferred to aggressive systemic and locoregional treatments [23].
Regarding an aggressive locoregional treatment in the setting of a metastatic BC, there are two different dilemmas to the presenting clinical scenario.
2.4.1 First, the Role of Aggressive Locoregional Therapy of the Primary Site in Women Presenting with Stage IV Metastatic BC
Using the data from the National Cancer Data Base (NCDB) of the American College of Surgeons (ACS) in a total of 16,023 patients with stage IV disease between 1990 and 1993, Khan and her colleagues showed in a multivariate proportional hazards model that women treated with surgical resection of the primary tumor with free margins compared with those not surgically treated to have superior 3-year survivals with a hazard ratio of 0.61 (95 % confidence interval, 0.58–0.65) [24]. The authors recommended that the role of local therapy plus systemic therapy in women with stage IV BC be compared with systemic therapy alone in a randomized trial. Since then, at least 14 cohorts have been published in the literature [25–37], including an analysis of 1988–2003 Surveillance, Epidemiology, and End Results (SEER) program data [38], to evaluate the role of the resection of primary site in the setting of the metastatic BC, almost all of which verified the findings of Khan et al. Five clinical studies (clinical trials or observational studies) regarding the issue are currently recruiting patients [39].
2.4.2 Second, the Role of Liver Resection for Metastatic BC to the Liver
The first report concerning major liver resection for metastatic BC in the literature dates back to 1963 on Alexander Brunschwig’s small cohort of patients from the Memorial Sloan Kettering Cancer Center (then the Memorial Hospital for the Treatment of Cancer and Allied Diseases) undergoing hepatic lobectomy for metastatic cancer including one patient receiving a left hemihepatectomy and partial [extra-anatomical] resection of the metastases to the right lobe due to BCLM years after the first operation for the primary tumor [40]. The patient survived 20 months after the liver resection. The author believed that the patients had achieved “although usually brief” palliation “in some instances” and that these operations [should be] “indicated essentially for palliation, and rather rigid criteria for selection of patients is necessary,” provided that “the metastases be macroscopically limited to one lobe of the liver.” A decade later, James H. Foster from Hartford, Connecticut, personally visited 98 hospitals to gather data on patients undergoing liver resection for solid tumors of all types, including secondaries. Together with his own small cohort, a literature review, and data from the Liver Tumor Survey (LTS), he presented a series of 345 operative survivors, about whom follow-up information was available [41]. The series included only five cases of BCLM, with one intraoperative death and a median postoperative survival of 6 months; 2-year survival was zero. The author described the results as “discouraging” and that the resection of liver metastases “cannot be recommended” except for the metastasis from a colorectal primary or from Wilms’ tumor. He, however, added that in cases of a long disease-free interval, the patient’s good general condition, the absence of accompanying diseases, and the surgeon’s experience and dexterity in hepatic resection, “liver resection may occasionally be indicated,” including the palliative resection of bulky tumors, functioning endocrine tumors, or in some rare cases to control hemorrhage from rupture of a necrotic tumor into the peritoneal cavity. In 1991, Elias et al. published the first series concerning specifically the liver resection for isolated BCLM [42]. From the 22 patients with BCLM undergoing explorative laparotomy, liver resection was possible in 12 patients, with a median survival time of 37 ± 9 months after resection. Although the median survival time was twice as much as those with conservative treatment, the authors considered the procedure as having “doubtful benefit” and rather serving as a “cytoreductive surgical treatment,” with the main limiting factor being the relatively low effectiveness of chemotherapy. They recommended that the chemotherapeutic protocols be radically modified.
Since then, many centers have published their experiences with the surgical resection for BCLM, some of which with unexpectedly promising results, trying to evaluate safety and effectiveness of liver resection for BCLM, to define indications for liver resection, to identify prognostic factors affecting survival, and to set management guidelines for surgical approach (Table 2.2) [4, 5, 43–71]. As shown in Table 2.2, through aggressive combination therapy including liver resection, these studies achieved median survivals ranging from 15 to 91 months, with 5-year survival rates ranging from 11 to 61 %. In our own series of 41 consecutive patients undergoing liver resection for BCLM between 1999 and 2008 at the Department of General, Visceral and Transplant Surgery at the Ruprecht-Karls University of Heidelberg, a median overall survival from the primary tumor of 211 months and a 10-year survival rate from primary tumor of 76 % could be achieved following liver resection [48]. Adam et al. [52] in a study of 454 patients undergoing hepatic resection for BCLM even succeeded to show a 10-year survival rate of 22 % after liver resection. Twenty one out of 29 studies report a postoperative mortality rate of 0 % (complication rate of 21 %; range, 0–44 %), suggesting that liver resection for BCLM can, in the majority of patients, be performed safely. Some series have yielded such promising results that Howlader et al. more recently claimed that the outcomes of liver resection for BCLM were comparable to those for colorectal cancer and suggested that liver resection be incorporated into the management guidelines [72]. It should be however pointed out that almost all of the studies mentioned in Table 2.2 are retrospective single-center case series of highly selected patients with limited hepatic metastases and no to minimal residual extrahepatic disease without a control group, thus allowing to yield only a level 4 evidence regarding the definitive role and survival benefit for the surgical resection of BCLM.
Table 2.2
Patient series evaluating outcome after liver resection for BCLM
Author | Country | yr published | Study period | n | Postoperative mortality (%) | Median survival (mo) | 5-yr survival (%) |
|---|---|---|---|---|---|---|---|
Weinrich [43] | Germany | 2014 | 2001–2007 | 21 | 0 | 53 | 33 |
Dittmar [44] | Germany | 2013 | 1997–2010 | 34 | 0 | 36 | 28 |
Mariani [45] | France | 2013 | 1988–2007 | 100 | 0 | 91 | 50 |
Polistina [46] | Italy | 2013 | 2004–2011 | 12 | 0 | 13.5 | 11.5 |
Abbott [47] | United States | 2012 | 1997–2010 | 86 | 0 | 57 | 44 |
Chua [5] | Australia | 2011 | 2000–2011 | 553 | 0 | 40 | 40 |
Hoffmann [48] | Germany | 2010 | 1999–2008 | 41 | 0 | 58 | 48 |
Thelen [49] | Germany | 2008 | 1988–2006 | 39 | 0 | 16–74 | 42 |
Caralt [4] | Spain | 2008 | 1988–2006 | 12 | 0 | 36 | 33 |
Lubrano [50] | France | 2008 | 1989–2004 | 16 | 0 | 42 | 33 |
Adam [51] | France | 2006 | 1984–2004 | 85 | 0 | 32 | 37 |
Adam [52] | France | 2006 | 1983–2004 | 454 | NR | 45 | 41 |
Martinez [53] | United States | 2006 | 1995–2004 | 20 | NR | 32 | 33 |
Sakamoto [54] | Japan | 2005 | 1985–2003 | 34 | 0 | 36 | 21 |
Weitz [55] | United states | 2005 | 1981–2002 | 29 | 0 | 48 | NR |
Yedibela [56] | Germany | 2005 | 1978–2001 | 24 | 7 | 33 | 43 |
d’Annibale [57] | Italy | 2005 | 1984–1999 | 18 | 0 | 32 | 30 |
Ercolani [58] | Italy | 2005 | 1990–2003 | 21 | 0 | 42 | 25 |
Vlastos [59] | United States | 2004 | 1991–2002 | 31 | 0 | 63 | 61 |
Arena [60] | Italy | 2004 | NR | 17 | NR | 36 | 41 |
Elias [61] | France | 2003 | 1986–2000 | 44 | 0 | 34 | 34 |
Carlini [62] | Italy | 2002 | 1990–1999 | 17 | 0 | 53 | 46 |
Pocard [63] | France | 2001 | 1988–1999 | 65 | 0 | 47 | NR |
Yoshimoto [64] | Japan | 2000 | 1985–1998 | 25 | NR | 34 | NR |
Selzner [65] | United States | 2000 | 1987–1999 | 17 | 6 | 25 | 22 |
Maksan [66] | Germany | 2000 | 1984–1998 | 9 | 0 | NR | 51 |
Kondo [67] | Japan | 2000 | 1990–1999 | 6 | 0 | 36 | 40 |
Raab [68] | Germany | 1998 | 1983–1993 | 34 | 3 | 27 | 18 |
Elias [69] | France | 1995 | 1986–1994 | 21 | 0 | 26 | 22 |
Lorenz [70] | Germany | 1995 | 1982–1991 | 8 | NR | 15 | 12 |
Schneebaum [71] | United States | 1994 | 1982–1990 | 6 | NR | 42 | NR |
More recently, Mariani et al. have shown for the first time in a case-matched control study in highly selected patients that medical treatment combined with surgical resection of BCLM appears to provide a survival benefit compared with medical treatment alone [45]. In the “medical treatment plus surgical resection” arm, only the patients with stable disease or disease responding to medical treatment were included. The patients had (except for bone metastases) no extrahepatic distant metastasis and had ≤4 resectable BCLMs. This group of patients (n = 51) was matched with the control “medical treatment alone” arm (n = 51) for age, year of the primary disease diagnosis, time to metastasis, TNM stage, hormone receptor status, and BC tumor pathology. The “medical treatment plus surgical resection” was associated with significantly improved survival (81 % vs. 51 % at 36 months, RR = 3.04, CI: 1.87–4.92, p < 0.0001). Hence, Mariani et al. have already upgraded the level of evidence for the effectiveness of surgical resection for BCLM from level 4 to 3b. However, this growing body of evidence should be further evaluated in terms of definitive survival and quality of life advantages through controlled randomized clinical trials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







