Fig. 12.1
Incision at the bladder neck
The longitudinal musculature at the prostate-vesical junction is then divided with scissors. This opens the space, which contains the vasa deferentia. The vasa are dissected, clamped and divided, followed by mobilization of the seminal vesicles bilaterally.
Care must be taken not to employ any source of energy along the tip and lateral surface of the seminal vesicle due to its proximity to the NVBs. A Hem-o-lok clip (Weck Closure Systems, Research Triangle Park, North Carolina) is placed to control the artery to the seminal vesicle, which is then transected with cold scissors. Denonvilliers’ fascia is incised horizontally close to the base of the prostate, entering the pre-rectal space along the posterior surface of the prostate (Fig. 12.2).
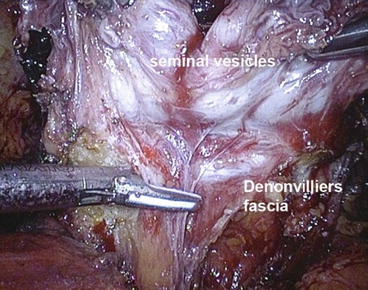

Fig. 12.2
Horizontal incision of Denonvilliers’ fascia
Both seminal vesicles and vas deferens, grasped by an Ellis clamp introduced through the 5 mm assistant port, are retracted antero-laterally to the left or right side, placing the respective lateral pedicle of the prostate on a gentle stretch.
Nerve sparing can be performed by intrafascial, interfascial or extrafascial techniques depending on patient selection and their individual pelvic characteristics. The dorsal vein complex is divided with UltraCision (Ethicon), but not ligated at this stage. Next the prostate apex is mobilized and both NVBs are gently dissected away from it. The urethra is sharply transected with cold endoshears and the specimen is entrapped in an endobag.
In order to improve continence, we reconstruct the posterior aspect of the rhabdosphincter, which mainly consists of connective tissue, using a Vicryl 1-0 stitch [26]. Then urethro-vesical anastomosis is completed with a 3-0 V-Loc (Covidien North Haven, CT, USA) running suture [27]. The anterior bladder neck is finally fixed to the puboprostatic ligaments and dorsal vein complex with a Vicryl 1-0 suture in order to reconstruct the anatomy and for haemostasis.
Oncological and Functional Results
Open RRP vs. LRP
Various reasons sited in support of LRP over open RRP include: faster recovery due to absence of larger surgical incision, less blood loss, better preservation of NVB and a better vesico-urethral anastomosis because of clarity of vision. The disadvantages of LRP according to the proponents of RRP include postoperative ileus, lack of proprioception leading to higher positive surgical margins, increased theatre space, increased cost of equipment, special surgical expertise, increased learning curve and use of electrocautery [28]. There is no clinical evidence to the commonly made assumptions that LRP is associated with less pain, better visualization during surgery and recover quicker than RRP [29]. As mentioned before there are no systematic clinical studies comparing the results of LRP and RALRP with open RP [30]. However various authors have made efforts to combine the data to get comparative information on all three procedures (Open vs. LRP vs. RALRP).
The outcomes after RP can be ranked as shown in the Table 12.1
Table 12.1
Outcomes of RP based on clinical importance
1. Oncological results: cancer control |
2. Periperative complications |
3. Postoperative complications: Early and late |
4. Urinary incontinence |
5. Erectile dysfunction |
6. Cost |
7. Blood loss |
8. Timing of catheter removal |
9. Length of hospital stay |
10. Postoperative pain |
11. Further operative treatments due to complications |
Oncologic Outcomes
The main parameter to assess the oncological outcome in RP is surgical margin positivity. A surgical margin is considered positive if tumour tissue or cells reach the ‘inked’ boundaries of excised prostate specimen. The risk of recurrence increases with positive margins, which in itself is a prognostic marker independent of grade, PSA, clinical staging and DNA ploidy. The long term follow up for LRP on its own is still unavailable, as it has been regularly performed only since late 90s. And also the data that are available tend to be mostly a combined data from different centers.
Margin Positivity
There appears to be not much difference in margin positivity rates between open and laparoscopic prostatectomy procedures [31]; however the results might be better with open procedure in pT2 disease [29]. Similar results have been reported by most of the non-randomized prospective studies, with the exception of a study by Roumeguere et al. [32], which reported a significantly higher positive surgical margin rates in patients undergoing RRP. However, when the data were stratified by the pathologic stage, no differences were found between the two procedures.
In studies comparing RRP and RALP, no significant differences were reported in the study evaluating the early phase of the RALP learning curve [33]. However, the largest prospective study demonstrated lower positive surgical margin rates in those patients treated by RALP, compared with RRP [34].
No differences were found between the results of LRP and RALP, considering both early phases [35] and more advanced phases [36] of the RALP learning curve. With regard to the comparison between LRP and RALP, no statistically significant difference was found in the positive surgical margin rates (RR: 0.97; 95 % CI of RR: 0.65–1.46; p = 0.9), even in a sensitivity analysis limited to pT2 cancers.
Blood Transfusion
Pure LRP allowed a significant reduction in blood loss and, consequently, lower perioperative transfusion rates compared with RRP. Similarly, transfusion rates were significantly lower in the patients having RALP compared with RRP. The results of LRP and RALP were similar with regard to blood loss and transfusion rates on a cumulative analysis [35].
Continence
Functional results seem to overlap between RRP and LRP, with 12-month continence rates ranging from 60 to 93 % after RRP and from 66 to 95 % after LRP in one study [35]. In a study by Hu et al. [37] the diagnosis for incontinence was lower for RRP patients than for RALP and LRP patients—12.2 versus 15.9 per 100 person-years, even after adjusting for baseline rates.
Sexual Function
In relation to sexual function no study, has shown any significant advantage of LRP over RRP in terms of erectile function recovery. The 12- and 18-month potency rates were similar, ranging from 10 to 93 % after RRP and from 42 to 76 % after LRP [35].
Tewari et al. [34] observed a faster recovery of continence and potency in patients who had RALP than in patients who underwent RRP. In another study on comparison of endoscopic techniques, there was no significant difference in outcomes of both RALP with LRP [36].
In summary LRP and RALP are followed by significantly lower blood loss and transfusion rates, but the available data are not sufficient to prove the superiority of any surgical approach in terms of functional and oncologic outcomes. Data concerning overall complication rates highlight that, once the learning curve is completed, LRP and RALP can be performed without a significant risk of major complications. Interestingly, our cumulative analysis showed a significant advantage in terms of overall complication rates in patients who underwent LRP in comparison with those treated by RRP. There were no significant differences between RRP and RALP. Like many others, we conclude that the critical issue in determining the risk of complications is the level of expertise of the surgeon and volume of work, regardless of the surgical approach. Further high-quality, prospective, multicentre, comparative studies are needed. It is important to compare the outcomes of endoscopic procedures with one of the oldest minimally invasive prostate procedure – perineal radical prostatectomy.
Cost
The overall service cost is an important factor in delivering any medical care. Both LRP and RALRP need continuing investment in expertise and advanced equipment. Consequently the two procedures cost much more than RRP. The robotic equipment cost about $1-2-1.5 million plus a yearly bill of more than $100,000 for maintenance. The cost of instruments per patient is estimated at $1,500 with an average total cost of $5,500. This is in comparison with the cost of LRP $3,870 and RRP $1,870 [38].
Kidney Cancer
Laparoscopic nephrectomy for T1-2 tumors has become a standard procedure within a short period of time. It should therefore be made available to the patients with kidney cancer. With the advent and widespread use of ultrasound and CT, there is an increase in the detection of small incidental renal tumors (incidentalomas). Nephron-saving surgery or partial nephrectomy would be more appropriate than radical procedure in these patients particularly those with T1 tumors. In patients with small tumors in addition to the size, other parameters such as the location of the tumor in the kidney and the patient’s general condition should be taken into account.
The principles of open surgery unequivocally apply to laparoscopic approach [39]. The decision making process for nephron saving treatments including surgery is discussed in the chapter on kidney cancer.
Radical Nephrectomy
The laparoscopic approach to radical nephrectomy (LRN) is considered a standard procedure for most patients with renal malignancy that are not eligible for a nephron-sparing procedure. The major advantages of LRN over open radical nephrectomy include decreased perioperative morbidity, lower blood loss, shorter length of hospital stay, and quicker convalescence.
Principles of the Technique
Retroperitoneal Radical Nephrectomy
Once the blunt dissection of loose hilar areolar tissue is performed, renal arterial pulsations are identified. The renal artery is circumferentially mobilized, clipped and divided. The renal vein is mobilized and controlled with a vascular stapler. A suprahilar dissection along the medial border of the kidney is performed up to the upper pole of the kidney. Adrenal vessels, including the main adrenal vein, are clipped and divided. Dissection is next redirected towards the superolateral aspect of the specimen, including en bloc adrenal gland, which is readily mobilized from the underside of the diaphragm. In the areolar tissue in this location inferior phrenic vessels to the adrenal gland are often encountered and need to be controlled. Care of the undersurface of the liver is taken on the right side.
The anterior aspect of the specimen is mobilized from the undersurface of the peritoneal envelope. The ureter and gonadal vein are secured, and the specimen is completely freed by mobilizing the lower pole of the kidney. The entire dissection is performed outside Gerota’s fascia, in keeping with standard oncological principles. An Endocatch bag (USSC, Norwalk, CT, USA) is introduced through the right-hand port incision, and the specimen is entrapped. Intact specimen extraction is performed through an appropriate muscle-splitting incision. Hemostasis is confirmed under lowered pneumoretroperitoneal pressure and ports are removed under direct vision. Fascial closure is performed for all 10 mm or larger port sites using a 2-0-vicryl suture.
Transperitoneal
The transperitoneal approach utilizes a 4-port technique. Overlying bowel is reflected, the colo-renal attachments are released, as well as the ligaments attached to spleen and liver, on the left and right side respectively. After exposing the Gerota’s fascia retracting the lower pole of the kidney helps to stretch the renal hilum. The major hilar vessels are then exposed, individually, clipped and divided, initially the artery and later the vein. The Endocatch bag is used, and the entrapped specimen is extracted intact through a muscle-splitting, low Pfannenstiel incision without morcellation.
Partial Nephrectomy/Nephron-Saving Surgery
Due to the increased usage of abdominal imaging techniques there seems to be increased detection of small renal tumors, which in turn has led to a greater utilization of nephron-saving surgical (NSS) techniques. In addition, NSS helps in preserving the overall renal function; the choice of treatment has to be individualized without compromising oncologic principles and outcome [40]. Various nephron-saving technique have been outlined in detail in the chapter on renal cancer.
The technique of laparoscopic partial nephrectomy for RCC has been in vogue for more than 15 years [41]. Initially, the resections were performed without ischemia, for small peripheral tumors. The introduction of warm ischemia has become a major concept in resecting more difficult lesions [42]. Subsequently it has become possible to excise the tumor accurately by laparoscopic procedures without massive hemorrhage, to close potential defects in the pelvicalyceal system, and to ensure hemostasis by suturing the cortical defect. The laparoscopic techniques of partial resection thus duplicate the open technique [43]. Improvements in suturing also include using absorbable clips, with marked reduction in warm ischemia time [44]. Shortening of warm ischaemic time could further be achieved by the “early declamping” technique [45]. Good hemostasis and closure of the pelvicalyceal system could also be achieved by running suture in the renal interstitial tissue. Bleeding vessels are selectively treated after release of clamps enhancing the reliability of hemostasis. The mean duration of ischemia could be reduced from 27 to 14 min. The principal outcome of these modifications is that ischemia time for laparoscopy is no longer than that for open surgery. We now routinely end warm ischemia after a maximum period of 20 min; any hemorrhage still present at this time can be easily controlled.
Cooling of the kidney permits longer periods of ischemia with no or minimal damage to the kidney. In open surgery cooling is achieved by encasing the kidney in crushed ice.
Similar technique could be employed in laparoscopic procedures also; however because of practical difficulties, it is not widely used [46]. However, a new type of soft ice (Gelice®, Fresenius Company, Germany) has been tested successfully in animal experiments [47]. Gelice® is easy to apply because of its consistency although it is currently not approved for use in humans.
Another option is to cool the kidney via a catheter in the pelvicalyceal system in a retrograde fashion via the ureter [48]. This type of cooling is not very effective because the small capacity of the renal cavity as the cooling fluid is lost easily if the renal cavity is opened for tumor excision.
Cooling the kidney by means of arterial perfusion through the clamped renal artery although effective is quite a laborious approach [49]. With this technique, the renal parenchyma is protected from toxic substances that accumulate during the clamping as they are washed out.
Cooling of the kidney is required when one anticipates a longer period of ischemia during excision of a complex tumor. Gill et al. [50] have modified his technique recently and now perform all laparoscopic partial resections with induced hypotension and without ischemia. Arterial blood supply to the kidney is visualized as much as possible, especially in cases of hilar tumors. Bleeding vessels are selectively coagulated. The results published so far have been promising. The technique is employed when using the conventional laparoscopic and robotic assisted approach.
In our unit we do carry out partial resections without ischemia wherever possible. Currently we are conducting at a prospective study using a new laser (Eraser 1,318 nm Rolle, Austria), which is not particularly effective in terms of cutting, but effective in achieving hemostasis.
Principles of Surgical Technique
The patient is placed in the standard lateral decubitus position as for nephrectomy. The assistant operates the camera and an additional instrument. The ports for lower-pole tumors are similar to those used for nephrectomy. The camera trocar is placed in the umbilical port. A 12-mm working port helps to rapidly insert and remove the tourniquets and suture materials. In obese patients the position of the umbilicus is likely to be displaced, which is taken into consideration while using umbilicus as a port. In obese patients the port for the optics is moved laterally to the border of the rectus muscle with other ports positioned accordingly. On the other hand, for upper pole tumors, the camera is placed on lateral border of rectus, above the umbilicus, and the working ports just below the costal arch. We use vascular ‘tourniquets’ to control blood vessels during partial nephrectomy. The sling is folded and passed through 10 mm silicon tube and then introduced through a 12-mm port. The looped end of the sling is passed around the vessel, and back into the end and tube (like attaching a luggage tag). The free ends of the vessel loops are then fixed with a large (purple) Hem-o-lock clip so that they cannot fall apart. Traction on the end of the vessel loop, with application of a second XL (gold) Hem-o-lock firmly against the end of the tube, causes vessel occlusion. The slings are colour coded-red for artery (Web sil loop maxi + 10-mm silicone tube) and blue for the vein (vascular silicone ties mini + 6-mm silicone tube).
Following the mobilization, the lower pole of the kidney is elevated (by the assistant via a lateral port). The pedicle is freed from surrounding structures, without skeletonization. The artery is left in situ within the surrounding connective tissue to reduce the risk of injury. A window is made between the pedicle and adrenal gland, through which a right-angle clamp (deflectable Snowden-Pencer, CareFusion, San Diego, CA, USA) is passed around the pedicle. With the help of this clamp, a self-made tourniquet folded over once to form a U-loop (vessel loop through 10-F silicon tube) is brought around the dorsal aspect of the pedicle. Next, the Gerota fascia is dissected from the kidney, so that adequate adjacent renal parenchyma is exposed. Intravenous mannitol (15 %w/v, 250 ml) is administered just before to the clamping. This is done by tightening the arterial tourniquet with a large Hem-o-lok clip (Fig. 12.3).
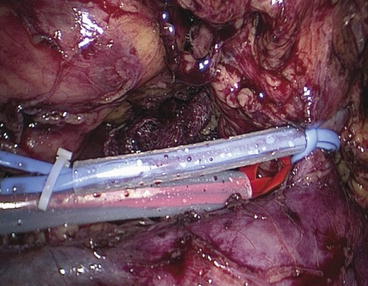

Fig. 12.3
Red tourniquet around the artery and the blue one around the vein
This clip is removed later to terminate ischaemia. The venous tourniquet can be used if there is a venous backflow. The renal capsule is incised about 5 mm away from the edge of the tumour by diathermy. The tumour is excised in a bloodless field, using cold Metzenbaum scissors to achieve a perpendicular cut through the parenchyma (Fig. 12.4). A close inspection of the cut surface while cutting helps to distinguish normal parenchyma from the tumour. If tumour tissue becomes visible or the surface is suspicious, excision of the specimen is continued within healthy tissue. Strict observation of this technique makes random biopsies of the excision bed unnecessary. As excision proceeds, vessels may be identified and clipped.
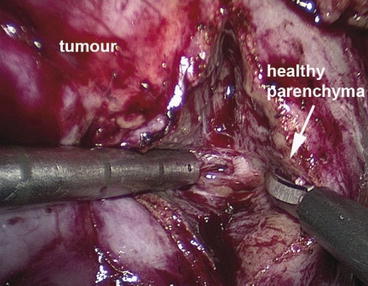

Fig. 12.4
The tumour is excised in a bloodless field
Reconstruction of the cut surface is performed in two layers. The inner part is closed in the region of collecting system by approximating the interstitial tissue using a running suture (2 × 3/0 Vicryl 15 cm Lapra-Ty with a knot at the end of the suture with a 26.4 mm 5/8 needle). This suture, which is secured on both sides with a resorbable Lapra-Ty clip to avoid time-consuming knotting, is started at the opposite edge of the resection bed. It includes arteries and veins, thereby providing a secure haemostasis. Care must be taken to avoid injury to large central vessels. The second layer running suture (Vicryl 1, M04 needle, 15 cm + 20 cm) is used through the whole thickness of the renal parenchyma. This running suture is secured with a large Hem-o-lock clip at the end of the suture.
A knot behind the clip prevents it from displacement or slipping through. The suture overruns a bolster of oxidized regenerated cellulose (Tabotamp/Surgicel, Ethicon 360, USA). The bolster is haemostatic by itself and provides haemostasis by tamponade with pressure on the vessels underneath in the interstitial layer. This suture is secured after each stitch with a large Hem-olock clip (Teleflex Medical) (Fig. 12.5). The ischaemia is terminated after the first stitch and placement of the collagen (Tabotamp-Ethicon) roll by releasing the tourniquet (although it remains in position). The Hem-o-lock clip is cut by means of an ultrasonic scalpel. This early declamping reduces ischaemia time is by about 10 min. It is never a problem to continue the parenchymal suture without ischaemia. The end of the running suture is secured with a Lapra-Ty clip in addition to the Hem-o-lock clip so that the suture cannot loosen. Fibrin glue (Tissucol Duo S Immuno, Baxter, Deerfield, IL, USA) is applied over the suture line and between the approximated edges of the parenchyma to avoid delayed bleeding.
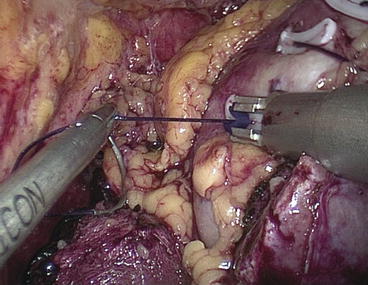

Fig. 12.5
The running suture is secured after each stitch with a large Hem-o-lock clip
Finally, the remnants of the Gerota fascia are re-approximated over the repair. The kidney is brought back into its normal position and attached to the lateral abdominal wall with one suture to avoid torsion. A drain is placed through the most lateral port. The specimen is removed through an extension of the lower abdominal port site incision.
Oncological Results
An important parameter is the rate of positive resection margin. This tends to vary between 1.8 and 2.4 % [51, 52]. The complication rates do not differ from those that are seen with open surgery. As the method is still quite new, published long-term data are scarce. Identical survival rates have been reported in a direct comparison with open surgery, spanning a follow-up period of 7 years. Tumor-free survival rates were 97.5 % (laparoscopy) and 97.3 % (open surgery), respectively [53].
Complications
In early publications, laparoscopy was reported to be associated with a higher rate of complications. Comparison of three clearly delineated time periods concerning a single surgeon showed that complication rates have fallen from initial 23.9 to 10.6 % [54]. This marked reduction is definitely not attributable to the learning curve of the surgeon alone, but also due to the evolution of the technique. In 18 patients with a complex hilar tumor, the rate of postoperative complications as well as the transfusion rate was 5.6 % [55]. It may be concluded that once the learning curve has been overcome, complication rates become similar to those for open surgery [56].
Comparison of Conventional Laparoscopy and the da Vinci® Robot
Very few studies published to date have directly compared two laparoscopic techniques (robot assisted and laparoscopic). Cho et al. [57] compared the data of the last ten patients operated on purely by the laparoscopic approach with the first ten patients operated on with the assistance of da Vinci® robot. The only difference is a longer period of ischemia in conventional laparoscopy (40 versus 31 min). However, certain aspects of this study have been criticized. The number of investigated patients was small. Although the size of the tumours was identical, no information was provided about the degree of complexity (location in the kidney, score). Furthermore, the period of ischemia in both techniques was markedly longer than that reported in the recent literature.
A direct comparison of partial resection by open surgery, conventional laparoscopy, and laparoscopy using the da Vinci robot was done in the USA and findings are interesting; a large number of parameters were considered in great detail [58]. There were minor variations in costs: conventional laparoscopy (10,311 $), open surgery occupied a mid-position (11,427 $), while the da Vinci® robot was very expensive (11,962 $).
Nephroureterectomy
Retroperitoneal approach: After the creation of the retroperitoneal workspace, the kidney is then retracted anterolaterally with a forceps, placing the renal hilum on traction. Gerota’s fascia is incised longitudinally in the general area of the renal hilum, parallel and 1–2 cm anterior to the psoas muscle. Blunt dissection in this avascular area of loose areolar fatty tissue is performed to identify renal arterial pulsations. The renal artery is circumferentially mobilized, clip occluded, and divided. The renal vein is mobilized and controlled with a gastrointestinal anastomosis vascular stapler. Suprahilar dissection is performed along the medial aspect of the upper pole of the kidney, and the adrenal vessels, including the main adrenal vein, are precisely controlled.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







