Chapter 19 Shailen Sehgal, MD; Phillip Mucksavage, MD Minimally invasive surgery via a laparoscopic or robotic-assisted approach has become the gold standard for many urologic procedures. While providing equivalent efficacy in a number of upper and lower urinary tract surgeries, laparoscopic procedures have a distinct advantage over open techniques in postoperative pain, cosmesis, recovery, and length of stay. A thorough knowledge of the basics of laparoscopy and laparoscopic anatomy is essential for any practicing urologic surgeon. A minimally invasive approach is considered the standard of care for radical nephrectomy for T1-T3a renal cell carcinoma, adrenalectomy for benign pathology, simple nephrectomy for benign disease, nephroureterectomy for urothelial carcinoma, dismembered pyeloplasty in adults, and pelvic lymphadenectomy. Urologic surgeries for which a laparoscopic technique is an acceptable alternative standard of care include partial nephrectomy, radical prostatectomy, and living-donor nephrectomy. In choosing patients for laparoscopic surgery, absolute and relative contraindications must be considered. Absolute contraindications to laparoscopic surgery are no different than for open alternatives (i.e., uncorrected coagulopathy, severe performance status/cardiopulmonary disease). There are a number of relative contraindications to laparoscopic surgery. However, all patients with a relative contraindication are amenable to laparoscopic surgery under certain circumstances. COPD: Typically, the abdomen is insufflated using carbon dioxide (CO2). Those with chronic obstructive pulmonary disease (COPD) are at danger of developing hypercarbia secondary to CO2 reabsorption after insufflation, but may benefit postoperatively from reduced narcotic requirements and improved pulmonary toilet. In patients with normal pulmonary function, this CO2 is easily removed from the body by the lungs. However, the lungs ability to do so in COPD is impaired, resulting in hypercarbia. Methods to decrease the CO2 reabsorption in those with COPD include changing the insufflation gas to helium and lowering intraabdominal insufflation pressure to 10-12 mm Hg. Pregnancy: This was previously believed to be a relative contraindication to laparoscopic surgery but now is not. Concerns were raised in regard to placental ischemia induced by pneumoperitoneum. These concerns were not corroborated in animal studies. In animal models, intraabdominal pressure (IAP) up to 10 mm Hg was considered safe; however, increasing the working pressure to 20 mm Hg produced maternal and fetal cardiopulmonary compromise. In fact, laparoscopic cholecystectomy and appendectomy are considered the standard of care during pregnancy. Obesity: Obesity was previously believed to be a relative contraindication to laparoscopic urologic surgery, but is no longer. In fact, the postoperative morbidity decrease from laparoscopic surgery is greater in the obese population as compared with the normal population. It has been shown, in upper urinary tract laparoscopic surgery, that patients with BMI greater than 30 kg/m2 had higher operative time, blood loss, and transfusion rates as compared with lean counterparts. However, major and minor complications were no different in the obese population. Conversion rates and analgesic requirements were also no different in lean versus obese patients. Previous abdominal surgery: Intraabdominal adhesions were previously thought to be a contraindication to laparoscopic surgery but are no longer. There are circumstances in which a laparoscopic lysis of adhesions (LOA) should not be undertaken, including peritonitis, hemodynamic instability, and coagulopathy. However, in the overwhelming majority of patients, laparoscopic LOA can be done with efficiency and safety, provided the surgeon is familiar with the technique. In fact, laparoscopic LOA has been shown to confer a quicker recovery of bowel function and fewer wound complications as compared with open LOA. Minimally invasive retroperitoneal approaches can also be utilized to avoid the potential adhesions seen after previous intraabdominal surgery. The patient is positioned in a 60- to 90-degree flank position with the operative side up. Decompression of the bowels and bladder via a nasogastric/orogastric tube and Foley catheter should be done prior to prepping the patient. The patient should be carefully padded at all pressure points, and an axillary roll should be placed to prevent brachial plexus injury. In regard to positioning on the bed, the break should lie between the twelfth rib and the anterior superior iliac spine once the patient is in flank position. The table should be flexed slightly, and the anesthesia team should properly bolster the patient’s head so it is not flexed downward. The lower leg should be flexed at the knee, and the upper leg should be straight. The patient may be secured to the bed using straps or tape. A “test roll” should be performed with operative personnel on both sides of the bed to ensure that the patient is properly secured. An arm board is placed on the side of the bed to which the patient is facing, and the arm should be placed palm down in a natural position with no tension. Once the above position is attained, the patient may be prepped (Figure 19-1). Retroperitoneal renal surgery requires the patient to be placed in a full flank position, and the table should be flexed as much as possible to facilitate retroperitoneal dissection. The patient’s ventral surface should be more towards the midline, rather than the edge, of the bed. This description will focus on robot-assisted laparoscopic prostatectomy (RALP). Positioning for laparoscopic prostatectomy is similar. The patient should be placed in a low lithotomy position to allow for the robot to be positioned between the patient’s lower extremities. Alternatively, split leg positioners may be used. The patient is placed in extreme Trendelenburg, allowing gravity to retract intraabdominal contents cranially. The patient’s buttocks should be brought to the end of the table, and the weight of the leg should be on the patient’s heel, which should be padded. The arms should be placed on either side and tucked, with the palms oriented so the thumbs are anterior. The elbows should be padded, and a strap should be placed over the patient’s chest. The strap should be placed so that a hand can be put between the strap and the patient’s body, but no tighter, to allow for adequate chest wall excursion. A Foley catheter should be placed after the patient is prepped, and a nasogastric/orogastric tube should be placed prior to prepping. Other laparoscopic urologic surgery positioning may be adapted from the above. For instance, robot-assisted and pure laparoscopic cystectomy or ureterectomy and reimplantation can be done from the same positioning as RALP, and robot-assisted and pure laparoscopic upper urinary tract surgery can be done from the same positioning as laparoscopic renal surgery (i.e., pyeloplasty can be done from the same positioning as transperitoneal laparoscopic renal surgery). Obtaining access to the peritoneal cavity is done primarily via two techniques (Veress [closed] or Hasson [open]) techniques. Each technique will be described in detail and both are safe and effective means of accessing and insufflating the peritoneal activity. The surgeon should choose the technique with which he or she is most comfortable. The Veress needle has a spring-loaded inner sheath. When the needle engages tissue, this sheath retracts, allowing the needle to puncture the tissue. Once the needle passes into the peritoneal cavity, the sheath deploys and guards the needle tip which greatly decreases the likelihood of injury to intraabdominal vessels and viscera. Safe access to the peritoneal space via the Veress technique is accomplished by employing the technique correctly. Three “clicks” are heard when the needle passes through the following layers on the anterior abdominal wall: skin, anterior rectus sheath, and posterior rectus sheath. Once the third “click” is heard, the needle should be held in this position. Rotating the needle could cause intraabdominal injuries if the needle is in the wrong position. The needle should then be aspirated, and the contents of aspiration should be examined. Succus or blood indicates the needle is in bowel or vasculature, respectively. If this is the case, the needle should not be removed. Instead, an alternate Veress needle puncture site should be chosen and the above technique repeated. After the initial aspiration, 2 cc of saline should be irrigated, and aspiration should be repeated. The needle should aspirate easily. The saline should quickly drop into the peritoneal space, provided the needle is in the correct location. The needle can then be connected to high-flow insufflation. The caliber of the Veress needle limits the inflow to no greater than 3 L/min. Attention should be paid to the pressure transduced, which should be less than 10 mm Hg. There are a number of reasons why the initial insufflation pressure may initially read greater than 10 mm Hg. If the needle is abutting an intraabdominal viscus, pressure may be elevated. In this circumstance, pressure may be reduced by retracting the abdominal pannus up, away from this viscus. In very obese patients, the weight of the abdominal wall may cause increased initial pressures, which will decrease with continued insufflation. If the abdomen is not uniformly insufflated, and the pressure persists greater than 10 mm Hg, the needle is in the preperitoneal space and needs to be advanced. The primary trocar can then be advanced via a blind or optical trocar (nonbladed). The laparoscope is then introduced to survey the abdomen for anatomic variation, adhesions, and damage to viscera or vessels from the Veress needle or initial trocar. Remaining trocars can then be placed under direct visualization. The Veress needle is removed, and the insufflation tubing is connected to the camera trocar. The first step in this technique is accessing the peritoneal space via a 12-mm or smaller incision. A Hasson or balloon trocar can be used. The trocar can be affixed to the fascia to ensure that it is not dislodged during the procedure. Insufflation is then connected to this trocar, and the abdomen can be surveyed. The sutures used to affix the trocar to the fascia can be used at the conclusion of the procedure to close the fascial defect. The Hasson technique should be used in children and pregnant patients. It should also be noted that current literature does not support the closed or the open technique as safer relative to the other. Pneumoperitoneum is traditionally attained using CO2 gas maintained at an IAP of 15 mm Hg. Animal studies have shown that increasing IAP to 20 mm Hg can result in impaired renal, cardiac, and pulmonary function. However, it should be noted that brief increases in IAP to 20 mm Hg appear to not be physiologically deleterious. In animal studies, impaired cardiac and pulmonary function at 20 mm Hg IAP was seen after 3 hours. The overall effect of pneumoperitoneum produces no change in cardiac output. Pneumoperitoneum compresses the vasculature, increasing peripheral arterial resistance and central venous pressure. Increased central venous pressure leads to decreased peripheral venous return. The heart rate increases in response to decreased preload, and cardiac output remains the same. However, arrhythmias are more common with pneumoperitoneum. CO2 induces hypercarbia and acidosis and increases the sympathetic response, all of which contribute to an increased incidence of arrhythmias. Pneumoperitoneum increases IAP. This increase in IAP is transmitted to the thoracic cavity. The result of the increased thoracic cavity pressure is decreased compliance, thereby increasing peak and mean airway pressures. Also, vital capacity is reduced. CO2 diffuses into the thoracic cavity, causing hypercarbia and acidosis. Hypercarbia induced by CO2 is usually inconsequential in those with normal pulmonary function. However, as mentioned previously, those with COPD may have difficulty eliminating this excess CO2. As such, arterial blood gases may be used to measure arterial CO2 saturations in this patient population. If hypercarbia develops, decreasing the IAP to 10 mm Hg and/or changing the insufflation gas to helium can reduce this effect. Many robot-assisted pelvic surgeries are done with the patient positioned in steep Trendelenburg. Trendelenburg positioning reduces chest wall compliance, impairing O2
Laparoscopic Surgical Anatomy, Laparoscopy, and Robotic-Assisted Laparoscopic Surgery
Introduction
Patient selection
Relative contraindications
Patient positioning
Renal surgery
Transperitoneal approach
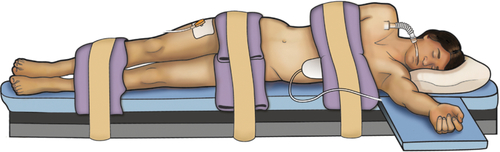
Retroperitoneal approach
Robotic-assisted laparoscopic prostatectomy
Other pelvic and upper tract surgery
Access
Closed (Veress) access
Hasson (open) access
Physiologic changes of pneumoperitoneum
Cardiovascular effects
Pulmonary effects
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



