Chapter 24 Eugene J. Pietzak, III, MD; F. Perry Wilson, MD MSCE An understanding of renal physiology helps provide a foundation for the practicing urologist to better understand the pharmacology, pathology, and therapies encountered in daily clinical practice. Urologists encounter issues pertaining to renal physiology and pathophysiology frequently, and this will only become more common as the prevalence of kidney disease nationwide increases with the aging population. It is the goal of this chapter to provide an overview of the basic concepts of renal physiology and pathophysiology for urologists. The kidneys receive approximately 20% of the cardiac output resulting in renal blood flow of approximately one liter per minute. Blood flow to the kidney proceeds sequentially from the renal arteries to interlobar arteries, arcuate arteries, interlobular arteries, and then to the afferent arterioles. Afferent arterioles develop into the glomerular tuft, a capillary network which is the site of all renal filtration. The glomerular tufts evolve into the efferent arterioles which go on to form the vasa recta which provide oxygenated blood to the renal tubules. The kidney is the only place in the body in which a capillary bed connects two arterioles — a necessary development to ensure adequate filtration pressure in the glomerulus. Renal blood flow is regulated by vascular resistance changes primarily of the efferent and afferent arterioles controlled by a complex interplay of neurohormonal signals in response to physiologic conditions. A single arteriole–glomerulus–tubule system is referred to as a nephron. Filtration of plasma across the glomerular membrane is the initial step in the formation of urine and the elimination of waste products from the blood. The fluid filtered through the glomerulus (and which will subsequently travel through the tubular system) is referred to as the ultrafiltrate. The glomerular filtration rate (GFR) is the sum of the GFRs of all individual nephrons and reflects overall renal function. GFR is determined by Starling forces. GFR = Kf [(hydrostatic pressure in glomerulus – hydrostatic pressure in Bowman capsule) – (oncotic pressure in glomerulus – oncotic pressure in Bowman capsule)] Kf is a coefficient accounting for glomerular membrane permeability and glomerular surface area. Because hydrostatic and oncotic pressure within Bowman space are relatively stable (and very low) under normal circumstances, GFR is determined primarily by the hydrostatic and oncotic pressure in the plasma. The permeability of the basement membrane is determined by characteristics of: (1) the fenestrated endothelial cells lining the glomerular capillary, (2) the glomerular basement membrane, and (3) the podocytes – epithelial cells surrounding the outside of the glomerular tuft. GFR is controlled by autoregulation, an incompletely understood process, which maintains renal blood flow across a wide range of systemic blood pressures. Autoregulation is mediated in part by myogenic mechanisms within the afferent arteriole. Feedback loops involving hormones and vasoactive substances also play a role. Autoregulation appears to be effective for the mean arterial pressure range of 40 to 70 mm Hg, but is less effective out of that range. Tubuloglomerular feedback also plays a significant role in regulating GFR. Tubuloglomerular feedback uses specialized cells within the macula densa of the distal tubule to sense the delivery of sodium and chloride and signal changes in efferent vascular resistance in response to changes in distal delivery. Measuring the actual GFR is not possible clinically, but GFR can be estimated by calculating the renal clearance of a substance that is freely filtered at the glomerulus and not secreted, reabsorbed, synthesized, or metabolized within the renal tubule. Clearance = (urinary concentration of substance) × (volume of urine over time) / (serum concentration of substance) Inulin or radiolabeled compounds can give the best estimate of GFR but are limited by the need for intravenous infusion at a constant rate and difficulty in measurement. Creatinine (Cr) clearance is the most commonly used surrogate for GFR in clinical settings. Creatinine is a breakdown product of muscle, which is produced at a fairly constant rate. Calculating the 24-hour creatinine clearance is a relatively simple way to estimate GFR. However, approximately 10% to 20% of creatinine is secreted in the proximal tubule, which leads to an overestimate of true GFR. Notably, as true GFR declines, the amount of creatinine which is secreted increases and can contribute up to 35% of total creatinine excretion. At best, creatinine clearance represents the upper limit of the true GFR. It is also important for clinicians to be aware that several medications, such as trimethoprim, inhibit the secretion of creatinine into the tubule, which decreases creatinine clearance without changing GFR. Creatinine levels also vary in settings of muscle loss or gain which also limits the accuracy of creatinine clearance. Other markers of renal function are under investigation. The blood urea nitrogen (BUN) concentration is too variable by the patient’s hydration and dietary protein intake to be clinically useful as a measure of GFR. Plasma cystatin C is a protein found in all nucleated cells that is produced at a constant rate and is unaffected by diet. Cystatin C is freely filtered, reabsorbed in the tubule system, and fully metabolized, preventing any reabsorption of cystatin C into the blood pool. Although cystatin C does have promise as a measure of GFR in the future, its use has been limited because serum levels are affected by acute disease processes, and cystatin C assays have limited availability. Formulas to accurately predict GFR without collecting a timed urine sample have been developed and are of significant clinical importance. The Cockcroft-Gault formula is a simple mathematical correction that estimates creatinine clearance with improved accuracy over plasma Cr alone by correcting for age, sex, body mass, and weight. The Cockcroft-Gault formula was originally calculated using normal healthy individuals, and therefore it is less accurate in individuals with impaired renal function. Cockcroft-Gault Formula for estimated GFR = (140 – age) × (body mass in Kg) × (0.85 of female) / (72) × (serum Cr in mg/dL). Estimating GFR using the Modification of Diet in Renal Disease formula (MDRD) is more complex but more accurate than the Cockcroft-Gault formula. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was developed to improve the MDRD formula, particularly at higher GFR. Both MDRD and CKD-EPI give an estimated GFR from serum creatinine, age, gender, and race. Because of inaccuracies in estimation of GFR at the higher range, many clinical laboratories will report all GFRs that are above 60 mL/min/1.73 m2 as greater than 60. Once the ultrafiltrate leaves Bowman space, it travels along a tubular system in which the properties of the fluid are modified by secretion and reabsorption of various dissolved substances and water. These processes are mediated by osmotic gradients, electrochemical gradients, and active transport. The proximal convoluted tubules (PCT) are the site of approximately 60% of reabsorption of the glomerular filtrate. About 65% of filtered sodium is reabsorbed by passive and active routes. About 90% of filtered bicarbonate is reabsorbed as well. Glucose is almost entirely reclaimed in the PCT; however, blood glucose levels above 200 mg/mL can exceed the transport threshold, resulting in glucosuria. The majority of filtered water, potassium, calcium, phosphate, and amino acids are also absorbed in the PCT. The loop of Henle reabsorbs about 25% of sodium via a sodium-potassium-two-chloride cotransporter (NaK2Cl). This transporter is inhibited by “loop” diuretics, such as furosemide. The loop of Henle helps establish a medullary interstitial osmotic gradient — a concentration of solute within the medulla of the kidney that, at 1200 mOsm/L, is four times that of the serum. This hypertonic milieu is necessary to allow for the concentration of urine in the collecting duct. In addition, the thick ascending limb is where Tamm-Horsfall mucoprotein production and secretion occurs. Tamm-Horsfall proteins aid in the prevention of urinary tract infections and form the matrix for all urinary casts. In the distal tubule, 5% to 10% of sodium is reabsorbed via the actions of an apical sodium-chloride cotransporter, which is inhibited by thiazide diuretics. About 10% to 15% of calcium is also absorbed via luminal channels. Calcium reabsorption is regulated mainly by parathyroid hormone (PTH), with higher levels of PTH increasing calcium reabsorption. Thiazide diuretics promote reabsorption of calcium and can be used as a medical treatment for calcium stone formers. The collecting tubule contains two main cell types, principal cells and intercalated cells. Principal cells are responsible for sodium reabsorption and potassium secretion. In the presence of aldosterone, an apical sodium channel is opened allowing reabsorption of sodium, with an attendant efflux of potassium into the filtrate to maintain electrical neutrality. The epithelial sodium channel is inhibited by amiloride (a potassium-sparing diuretic). The collecting tubule is only permeable to water in the presence of antidiuretic hormone (ADH). When ADH is present, apical aquaporin channels are inserted into principal cells, allowing the efflux of water from the filtrate towards the hypertonic interstitium. ADH is released from the supraoptic nucleus of the hypothalamus in response to increased serum osmolality or hypovolemia. Collecting tubule intercalated cells are responsible for acid handling. Type A intercalated cells actively secrete protons, whereas type B intercalated cells secrete bicarbonate into the filtrate. The relative magnitude of these processes will vary, depending on serum and cellular pH, and in general work to preserve a systemic pH of 7.4 (Figure 24-1). Serum sodium concentration reflects both the total body sodium and total body water content. Perturbations in serum sodium concentration are nearly uniformly due to disturbances in water handling, rather than overconsumption or overexcretion of sodium. 2. Hypernatremia
Renal Physiology and Pathophysiology
Introduction
Renal physiology
Glomerular filtration
Calculating glomerular filtration rate
Tubular physiology
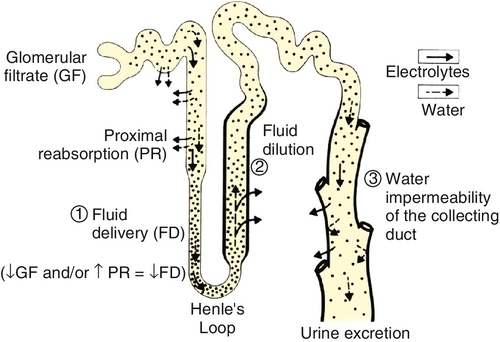
Sodium and water balance
Hypernatremia is due to loss of water in the absence of adequate water intake. Because of the powerful effect of thirst on behavior, individuals with large water losses from urine and stool rarely become hypernatremic unless they are deprived access to water or have an impairment of thirst (as can be seen in dementia). The underlying cause of hypernatremia is best determined by volume status.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


