Chapter 16 Thomas J. Guzzo, MD, MPH; S. Bruce Malkowicz, MD; David J. Vaughn, MD; Alan J. Wein, MD, FACS, PhD (hon) Prostate cancer is the most common cancer in men and the second greatest cause of cancer mortality in men. One in six men will be diagnosed with prostate cancer; however, only one in 36 will die from prostate cancer. Prostate-specific antigen (PSA) screening has led to a dramatic stage shift over the last two decades, with most men currently presenting with low-risk, organ-confined disease. PSA screening has also led to an increase in the detection of indolent prostate cancer as evidenced by the discrepancy between lifetime risk of prostate cancer diagnosis compared to that of prostate cancer mortality. Early detection likely improves outcomes in a subset of men; however, overtreatment of indolent cancer is now recognized as a potential drawback of screening. For those with localized disease, definitive therapy provides excellent oncologic outcomes. For those patients with intermediate or high-risk cancer for whom the risk of disease recurrence after local therapy is significant, combined modality therapy may be beneficial. The nature of biochemical recurrence after local treatment is being better defined, allowing for the stratification of these patients. Treatment of advanced disease has classically been provided through some form of androgen ablation and cytoreductive chemotherapy; however, recent advances in the treatment of locally advanced and metastatic prostate cancer are likely to redefine our treatment paradigms in this patient population moving forward. In 2013 there were an estimated 238,590 new cases of prostate cancer and approximately 29,720 cancer-related deaths in the United States. Worldwide there are approximately 899,000 new cases per year with 258,000 deaths, making it responsible for almost 12% of all new cancers and nearly 6% of cancer deaths. After the rapid increase in detected cases after the introduction of PSA testing, the number of incident cases has decreased to its present level. The death rate from prostate cancer has decreased by approximately 25% in the past decade, yet it cannot be said with certainty at this time that this is the result of earlier detection and therapy. PSA screening has been in widespread use in the United States for two decades. While we have definitely noted a stage migration toward that of lower risk, clinically localized disease, the benefit of screening remains controversial. Recently the results of two large randomized PSA screening studies have been reported with conflicting results. The Prostate, Lung, Colorectal, and Ovarian (PLCO) screening trial randomized 76,693 men in the United States to receive annual prostate cancer screening versus usual care. After 7 to 10 years of follow-up, there was no difference in prostate cancer mortality between the screened and unscreened groups. However, the methodology of this study has been heavily criticized. Notably, there was a significant (53%) contamination rate in the control group in addition to 40% of men undergoing at least one PSA test prior to randomization. Furthermore, the biopsy rate was only approximately 40% in men who were positively screened. The European Randomized Study of Screening for Prostate Cancer (ERSPC) randomized 162,387 men from seven European countries to receive PSA screening versus no screening. With a mean follow-up of 8.8 years, a 20% relative risk reduction from prostate cancer mortality was reported in the PSA-screened group. The number needed to screen to prevent one prostate cancer death was 1,410, and the number needed to treat was 48; however, these numbers have proven to be lower with greater follow-up. The negative findings in the PLCO study, the high number needed to treat in the initial ERSPC study, and the potential morbidity associated with prostate biopsy and definitive treatment led the U.S. Preventive Services Task Force (USPSTF) to recommend against population-based PSA screening for prostate cancer. The USPSTF recommendation has sparked much debate both within and outside of the urologic community. The American Urological Association (AUA) has also recently updated their clinical guidelines on prostate-cancer screening. The AUA has made the following recommendations: 1. PSA screening in men under the age of 40 is not recommended. 2. Routine screening in men between the ages of 40 to 54 years at average risk is not recommended. 3. For men 55 to 69 years old, the decision to undergo PSA screening involves weighing the benefits of preventing prostate cancer mortality in 1 man for every 1000 and screened over a decade against the known potential harms associated with screening and treatment. For this reason, shared decision making is recommended for men age 55 to 69 years who are considering PSA screening, and proceeding based on the patient’s values and preferences. 4. To reduce the harms of screening, a routine screening interval of 2 years or more may be preferred over annual screening in those men who have participated in shared decision making and decided on screening. As compared to annual screening, it is expected that screening intervals of 2 years preserve the majority of benefits and reduced overdiagnosis and false positives. 5. Routine PSA screening is not recommended in any man over age 70 or any man with less than a 10 to 15 year life expectancy. Prostate cancer is distributed in a very uneven manner with regard to race. Black Americans have the highest mortality rate from this disease, which is also highly prevalent in the Caribbean and Africa. There is a significant contrast to native Asian men who have the lowest disease incidence and death rate from this condition. Although lower than in other ethnic groups, prostate cancer incidence and disease mortality demonstrate an upward trend in Asian countries. Caucasian men in the United States and Europe have an intermediate rate of disease expression, with the highest incidence rate and disease mortality found in northern Europeans. 2. Family history: A twofold greater risk for developing prostate cancer exists in those individuals with a first-degree relative with prostate cancer. This climbs to almost ninefold if three first-degree relatives are affected. Risk also exists if second-degree relatives have a diagnosis of prostate cancer. Alterations in specific genes or genetic loci may contribute to the development of disease. Hereditary prostate cancer is usually defined as multiple affected family members and a distribution along several generations. Approximately 20% of patients may have a familial association with another individual with prostate cancer. 3. Geography: Data suggest an inverse relationship between latitude and the incidence of prostate cancer. Northern populations have a greater level of disease, and it is hypothesized that it is related to the lower vitamin D levels secondary to less exposure to ultraviolet radiation. This may also be due to dietary changes or other factors. Native black populations in Zaire have lower levels of prostate cancer compared to ethnic Zairians living in Belgium. b. Recently chromosomal rearrangements similar to that seen in certain sarcomas have been identified in prostate tumors. Gene fusions resulting in androgen-driven oncogenic gene products suggest a new method of tumor pathogenesis. This is exemplified in the fusion/rearrangement of TMPRSS2 with ETS transcription factor genes. TMPRSS2-ETS fusion has been identified in up to 50% of PSA-detected tumors. The utility of TMPRSS2-ETS fusions as a prognostic marker is yet to be elucidated but is under investigation. c. Androgen receptor CAG repeat length, alterations in SRD5A2 (5-alpha reductase-2), and cytochrome p450 genes associated with steroid metabolism (Cyp 3A4, Cyp 19A1, Cyp 17A1) have some relative impact on prostate cancer susceptibility alone or in concert. d. Data suggest that the GSTM1-null phenotype may increase prostate cancer risk in smokers. 2. Androgens: Studies on androgen levels have been conflicting but have suggested higher testosterone levels or dihydrotestosterone/testosterone ratios in black American men. Exposure to androgens at certain developmental time periods is likely critical in prostate cancer carcinogenesis. Mutations or alterations in the androgen receptor (CAG repeats) may also be unequally distributed among individuals in populations, affecting response to testosterone. 3. Diet: High intake of animal fat is associated with increased prostate-cancer risk. Conversely the intake of soy products (isoflavonoids, phytoestrogens, Bowman-Birk inhibitor) may affect the development and progression of prostate cancer. Migration studies of Asians moving west demonstrate an increase in prostate cancer incidence compared to that of Caucasian populations in the same area. Studies in other tumor systems on vitamin E and selenium showed a one-third to two-third decrease in prostate cancer incidence, respectively. However, studies specifically designed to evaluate the effect of vitamin E and selenium in prostate cancer have not demonstrated a preventative association. 4. Insulin like growth factor (IGF) system. These are peptides similar in structure to proinsulin that modulate proliferation, apoptosis, and tissue repair. IGF-1 and IGF-2 combine with one of six binding proteins (usually IGFBP3). Several studies have demonstrated that elevated serum levels of IGF-1 are associated with a higher risk of prostate cancer. The relative impact of the IGF axis on prostate cancer development or detection is currently unclear. 5. Inflammation: A growing body of investigation suggests that chronic inflammatory processes may play a role in the pathogenesis of prostate cancer. A pathway from normal tissue to proliferative inflammatory atrophy (PIA), to prostatic intraepithelial neoplasia (PIN), with resultant invasive prostate cancer has been proposed. The proposed pathway from inflammatory atrophy to invasive disease is a working hypothesis under investigation. Evidence for high-grade PIN as a precursor of invasive disease has grown stronger with further genetic and biochemical research. PIN is the proliferation of the acinar epithelium, and high-grade PIN is associated with the subsequent diagnosis of prostate cancer in approximately 25% of patients. Although the natural history of this condition is incompletely defined, patients should be counseled regarding the association between high-grade PIN and prostate cancer, and repeat prostate biopsy should be considered within one to three years after an initial diagnosis of high-grade PIN. 2. Mucinous variant: If more than 25% of the representative tissue has mucin-containing glands, this designation may be given. It is purported to have an equal or worse outcome than classic adenocarcinoma. 3. Signet cell carcinoma: This is another adenovariant with rapid progression and poor prognosis. 4. Endometrioid or ductal carcinoma: A variant that usually presents at advanced stage with papillary-like growth; ductal carcinoma generally has a poor prognosis. 5. Small cell carcinoma: A neuroendocrine variant of prostate carcinoma, this often presents with normal PSA values. Although the general prognosis is poor, it may respond to platinum-based therapy. 6. Urothelial carcinoma: This may arise from the distal prostatic ducts. It is usually manifest as an extension of primary bladder cancer. Stromal invasion carries a poorer prognosis than ductal infiltration. 7. Prostate sarcoma: This is a very rare tumor, generally designated as leiomyosarcoma. Recent data demonstrate c-kit positive staining in several cases suggesting potential therapy with Gleevec. 8. Hematologic malignancies: Leukemia and lymphoma variants are rare. 9. Metastases: Malignant melanoma, colorectal carcinoma, and pulmonary metastases have been documented. 2. Staging is generally designated by the TNM system (Tables 16-1 and 16-2). This provides a general description of tumor extent, yet may not fully represent the degree of tumor volume or the significance or insignificance of microscopic tumor extension beyond the prostatic capsule. Often the designation of organ confinement, extracapsular extension, and the degree of margin positivity, with some sense of the overall tumor volume, better describes lesions that are similar yet might be categorized more divergently. Table 16-1 TNM Classification Table 16-2 TNM Definitions PSA, Prostate-specific antigen. Screen-detected prostate cancers are typically asymptomatic. In more advanced stages, it may be associated with urinary obstructive symptoms or hematuria. Bone pain is common in those patients with metastatic disease. A nodule on the prostate or induration of the gland is a hallmark sign on physical examination. It is not always specific for a carcinoma and can underestimate the extent of disease when it does represent a carcinoma. 2. The data from the Connecticut tumor registry provide reasonable information for natural history and depict significantly worse outcomes in those individuals with higher Gleason scores. 3. Follow-up from Scandinavian, non-PSA screen-detected patient cohorts have reported a 33% rate of metastatic progression and 21% rate of prostate cancer mortality at 15 years for patients undergoing watchful waiting. 4. In patients with classic metastatic bone disease, the median survival is 27 to 33 months. Mortality is 75% at 5 years and 90% at 10 years. Recent data suggest that androgen-independent prostate cancer in the presence of metastatic disease has a median survival of 16 months. 2. Transrectal ultrasound (TRUS): Multiple studies have demonstrated that TRUS is sensitive yet not specific for the detection of prostate cancer. The classic finding is a hypoechoic lesion that in general has a 30% chance of being positive for carcinoma. Prostate cancer can also present as an isoechoic or hyperechoic lesion. The most important use of TRUS is in aiding transrectal guided needle biopsies of the prostate gland. 3. Serum markers: The classic marker for prostate cancer was acid phosphatase. The enzymatic test was elevated in 70% of patients with extracapsular and metastatic prostate cancer. The more sensitive radioimmunoassay of this enzyme is not as useful for detecting extracapsular disease and has no value as a screening test for prostate cancer. PSA is the most useful marker in the detection and monitoring of prostate cancer and is discussed in detail separately. 4. Prostate needle biopsy: Prostate biopsy is most commonly performed via a transrectal ultrasound guided approach. Contemporarily 10 to 12 cores of tissue are obtained. The procedure is generally well tolerated; however, biopsy-associated complications include hematuria, hematospermia, hematochezia, urinary retention, and, rarely, sepsis. Postbiopsy sepsis is becoming more common with the emergence of fluoroquinolone-resistant bacteria. The percentage of positive cores, line length, or line percentage of cancer per core can provide further predictive information with regard to staging and outcomes. 5. Bone scan: Before a lesion can be seen on a conventional radiograph, it must have replaced bone mass by 30% to 50% and be 10 to 15 mm in diameter. Plain film correlates of suspicious areas are often obtained. In some cases dedicated magnetic resonance imaging (MRI) or computed tomography (CT) imaging can resolve equivocal cases. Patients with a PSA value less than 10 ng/mL rarely demonstrate metastases. 6. CT and MRI: CT scans of the pelvis have poor performance characteristics for assessing metastatic disease and are not part of standard staging. Body coil MRI is performed similarly. Endorectal MRI may provide additional staging information in those patients with intermediate PSAs and greater than 50% positive needle-core biopsies. Additionally, data obtained by MRI spectroscopy may provide further information regarding the location and nature of malignant lesions. 7. PSA b. Physiology: PSA liquefies the seminal coagulum that is formed after ejaculation. A substrate produced in the seminal vesicles has been identified. PSA has activity like chymotrypsin and trypsin. The half-life of PSA is 2.2 to 3.2 days. c. Marker properties: There is no PSA level that indicates zero risk of having prostate cancer. Initially a PSA value of 4.0 ng/mL was used as a cut-off point for biopsy. A value above 2.6 ng/mL in younger men has also been used as a trigger for biopsy. Serum values are not generally altered by DRE but can be affected by recumbency, urologic instrumentation, ejaculation, and prostate biopsy. Nonmalignant conditions that affect PSA levels include prostatitis, prostate infarction, and benign prostatic hypertrophy (BPH). d. General clinical use: The principle use of PSA is in disease detection. The most specific use for PSA is the monitoring of patients after radical prostatectomy. Postoperative baseline values should be in the undetectable range. Residual disease is suggested by any detectable postoperative levels, and values greater than 0.2 ng/mL suggest biochemical failure of therapy. It is also used to monitor the response to radiation therapy. 8. Other markers: The lack of disease specificity associated with serum PSA testing has led to investigation of more specific markers for prostate cancer. PCA3, which is a urine-based biomarker, has been shown to have increased expression in 95% of prostate cancer cases. PCA3 testing has recently been approved by the U.S. Food and Drug Administration (FDA) in the setting of an elevated PSA in men who have previously had a negative prostate needle biopsy. As discussed previously, recent discoveries in gene fusions have led to intense investigation in the utility of such markers; however, this is still considered investigation. Circulating tumor cells (CTCs) have also been investigated both in the diagnostic and therapeutic response setting. The FDA recently approved the use of CTCs in monitoring disease response in patients with metastatic prostate cancer. 2. 5-alpha-reductase inhibition: A major phase III chemoprevention trial, the Prostate Cancer Prevention Trial (PCPT), demonstrated a 24.8% reduction in period prevalence of prostate cancer in patients using 5 mg of finasteride (type II) daily compared to placebo. However, the prevalence of higher grade prostate cancer was 25% higher (6.4% versus 5.1%), dampening this overall positive result. The reasons for this increase in higher grade disease remain to be fully explained but have hindered widespread adoption of 5-alpha-reductase inhibition as chemoprevention. The REDUCE trial using dutasteride, a type I and type II 5-alpha-reductase, also demonstrated a reduction in prostate cancer detection (23% compared to placebo). 3. Toremifene: This agent is a synthetic estrogen receptor modulator (SERM) that in phase IIb/III trials demonstrated the ability to reduce the progression of those patients with high-grade PIN to prostate cancer by 22%. A more recent phase III trial including men with a history of high-grade PIN did show a 10.2% relative risk reduction for prostate cancer compared to placebo; however, it was not statistically significant. 4. Selenium and vitamin E: In large phase III chemoprevention trials targeting other primary tumors, a secondary end-point analysis demonstrated that selenium reduced the incidence of prostate cancer by 63% in a study targeting skin cancer, and vitamin E reduced prostate cancer by 32% in a lung cancer study. This provided the basis of the SELECT trial. This trial was discontinued early as it clearly demonstrated no risk reduction with either selenium or vitamin E supplementation. 5. Lycopene: This agent is a water-soluble antioxidant. There are several lines of evidence but no conclusive proof that it may have an impact on prostate cancer chemoprevention. 6. Antiinflammatory agents: General nonsteroidal antiinflammatory drugs (NSAIDs) and cyclooxygenase (COX)-2 inhibitors demonstrate antiprostate cancer activity in the laboratory but are not under current consideration for study as chemopreventive agents because of their additional side effects. 7. Soy and other isoflavones: The components of soy may have anticancer properties, and epidemiologic studies support the potential for risk reduction by the inclusion of these substances in the diet. Several statistical approaches to the stratification of patients with regard to outcomes based on clinical data have been developed. Approaches using multiple regression analysis, nomograms (Figure 16-1), and neural networks have been employed for several different scenarios for prostate cancer. Hybrids of these methods have also been tested. In general the correlation of these models to individual outcomes is in the 0.7 to 0.75 range. Nomogram strategies may provide more individualized information. All of these methods may be useful in better directing patient choices and the use of additional therapies in the future. 2. Watchful waiting: This approach has generally been reserved for men with significant comorbidities that limit their overall survival and those unwilling to consider other forms of treatment. With 15-year follow-up, the SPCG-4 trial of surgery versus watchful waiting demonstrated improved outcomes for those undergoing radical prostatectomy including metastasis rate (11.7% risk reduction), disease-specific survival (6.1% reduction), and overall survival at 10 years (6.6% reduction). These findings were more pronounced in patients younger than the age of 65 years. 3. Active surveillance: With the significant stage shift in prostate cancer, the prevalence of low-grade, low-volume cancer has increased significantly. It has been postulated that the majority of very low risk prostate cancers pose little threat to an individual’s mortality. In light of this, active surveillance has become an increasingly popular option for men with low risk disease in an attempt to minimize the morbidity of treatment in those who are unlikely to die from prostate cancer. Active surveillance differs from that of watchful waiting in that there is strict surveillance by periodic DRE and PSA and repeat prostate biopsy, with the intent for curative intervention with progression to more aggressive disease characteristics. While active surveillance criteria vary from center to center, generally men considered appropriate candidates have: Gleason score less than or equal to 6 disease; less than or equal to 2 positive biopsy cores; less than or equal to 50% of any core involved with cancer; a PSA of less than or equal to 10; and a PSA density of less than or equal to 0.15. Classic triggers for intervention are volume or grade progression on subsequent biopsy. PSA doubling time has also been used by some as an indication for intervention. Approximately 30% of men in the Johns Hopkins active surveillance cohort ultimately underwent therapeutic intervention (roughly three quarters based on biopsy reclassification). With intermediate term follow-up, active surveillance has been reported to be a safe option for highly selected men with low-risk disease. 4. Radical prostatectomy: This is an appropriate treatment choice for younger patients and for older patients who are very fit and desire this form of treatment. Generally, a chronologic age of 70 is used as a relative cutoff point, but decisions need to be individualized with respect to health status and anticipated life span. Surgical mortality is less than 0.2% but 1% to 2% of patients may experience a pulmonary embolus or deep vein thrombosis. Surgical approaches include retropubic, perineal, robotic, and laparoscopic. Urinary incontinence may occur in up to 10% of patients. The potential for erectile dysfunction (ED) can be decreased with surgical approaches that spare the cavernosal nerves; however, age and preoperative erectile function are also significant predictors of ED following surgery. A bladder neck contracture can occur in 2% to 3% of patients. Disease-specific survival is in the 95% range over 10 years. 5. Radiation therapy b. Interstitial brachytherapy: Ultrasound-guided transperineal brachytherapy has become an accepted modality for the treatment of localized prostate cancer. It is difficult to treat large prostate glands (greater than 50 cm3) with this technique. Intermediate term results (10 years) suggest similar outcomes to surgery or EBRT in selected patients. The usual radiation sources are 125I and 103Pd. The principle urologic side effect is voiding dysfunction, which is usually short term but may be persistent. Those individuals with high International Prostate Symptom Score (IPSS) scores should not be considered for this technique. 6. Cryosurgical ablation of the prostate: This technique has undergone multiple modifications and is currently performed with small-caliber needles using freeze-thaw cycles employing argon and helium gas. More recent series show less morbidity and a decrease in chronic pelvic pain noted in early reports. While cryotherapy is of controversial utility in the primary setting, it has shown value in salvage cases for local recurrence after radiation therapy. 7. High-intensity focused ultrasound (HIFU): HIFU is currently under investigation for treatment of focal and localized disease. Early data demonstrate feasibility, yet data on intermediate- and long-term outcomes and side effects are pending. HIFU is not currently FDA approved in the United States for primary treatment. 8. Posttreatment follow-up: Serum PSA is the single most important follow-up parameter in evaluating posttreatment patients with either surgery or radiation. b. In general, response to radiation therapy is predicated on the pretreatment PSA and can be predicted by the posttreatment PSA. The closer to an undetectable value post treatment the better the overall outcome. c. There is a role for radiation therapy after postsurgical biochemical failures. Success is best when therapy is instituted before the PSA is greater than 1.0 ng/Ml, and studies have also demonstrated improved therapeutic responses at even lower PSAs. Percentage for cure ranges between 30% and 50% in different series. Salvage surgery can be employed in radiation therapy failures. 2. PSA failure in surgery: This definition ranges from any detectable PSA to 0.4 ng/mL. Low, stable detection of PSA may occur in postoperative patients and is often attributed to persistent benign tissue. Once the PSA value rises above 0.2 ng/mL, it rarely recedes. This value is therefore considered an absolute threshold for biochemical failure. 3. PSA failure in radiation patients: A strong definition for these patients has been more difficult to define due to the persistence of PSA detection after therapy and the kinetics involved in defining true progression from variations about a mean value. Nadir value plus two consecutive rises is the current operational definition, yet other definitions may be employed in the future. 4.
Adult Genitourinary Cancer
Prostate and Bladder
Prostate cancer
General considerations
Incidence
PSA screening
Epidemiology
Etiology
Pathology
Grading and staging system
TNM 2002
Histologic Description
Tis
Carcinoma in situ
Ta
Epithelial confined, usually papillary
T1
Invading lamina propria
T2a, b
Invasion of the muscularis propria:
2a: superficial invasion
2b: deep invasion
T3a, b
Perivesical fat invasion:
3a: microscopically
3b: macroscopically
T4a, b
Invasion of contiguous organs:
4a: prostate, vagina, uterus
4b: pelvic sidewall, abdominal wall
NO
No lymph node involvement
N1
Single ≤ 2 cm
N2
Single > 2 cm, ≤ 5 cm
Multiple ≤ 5 cm
N3
Single or multiple > 5 cm
M0
No distant metastases
M1
Distant metastases
TNM 2002
Definitions
T1
Tumor an incidental histologic finding
T1a
< Gleason score 7
< 5% of tissue resected
Tumor an incidental histologic finding
T1b
> Gleason score 7
> 5% of tissue resected
T1c
Tumor identified by needle biopsy (e.g., for elevated serum PSA)
T2
Tumor confined within the prostate
T2a
Tumor involves one lobe or less
T2b
Tumor involves more than one lobe
T3
Tumor extends through and beyond the prostate capsule
T3a
Unilateral extracapsular extension
T3b
Bilateral extracapsular extension
T3c
Tumor invades seminal vesicle(s)
T4
Tumor is fixed or invades adjacent structures other than seminal vesicles
Signs and symptoms
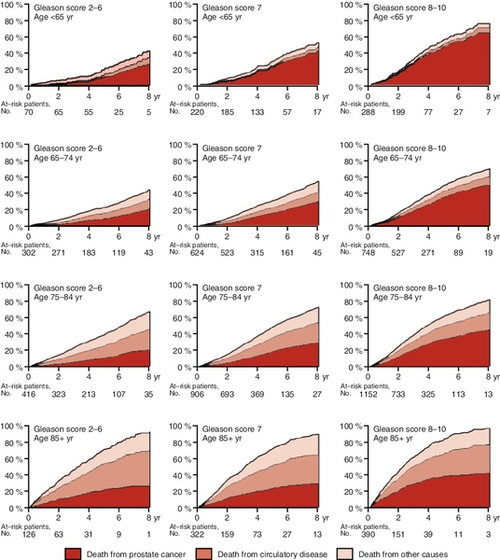
Natural history
Diagnosis and staging/PSA screening
Cancer prevention
Outcome prognosis and stratification
Treatment of localized disease
Treatment of biochemical and clinical failure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



