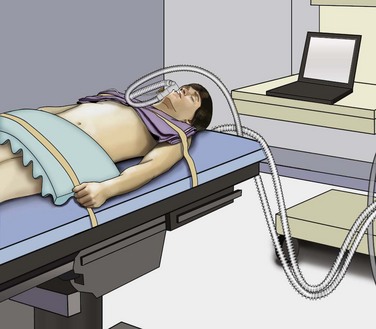
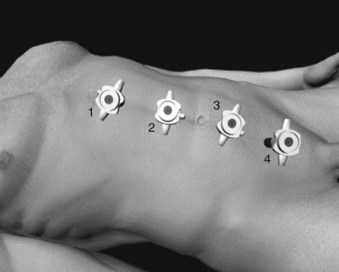
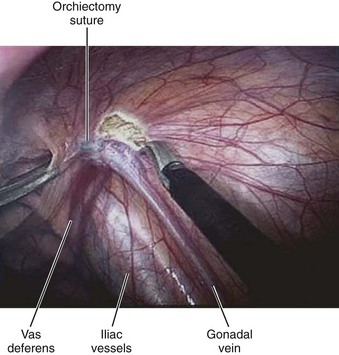
Mohamad E. Allaf, MD, Louis R. Kavoussi, MD, MBA Germ cell tumors (GCTs) are the most common malignancy in men between the ages of 15 and 35 (Carver and Sheinfeld, 2005). Fortunately, testicular cancer is also one of the most curable solid organ neoplasms, owing in large part to an excellent multimodal treatment paradigm that includes effective platinum-based chemotherapy and surgery (Einhorn, 1981). Although contemporary survival rates for GCTs are more than 90%, it is critical to note that cure rates and patient morbidity depend on selection of the management options. Retroperitoneal lymph node dissection (RPLND) plays a major role in the management of patients with GCTs. The role of surgery continues to evolve owing to advances in chemotherapy regimens, clinical staging modalities, and continued surgical innovation (Sheinfeld and Herr, 1998; Allaf et al, 2005; Albers et al, 2008). Whereas primary chemotherapy is favored in Europe, traditionally RPLND has been the management strategy of choice in the United States for high-risk patients with clinical stage I nonseminomatous GCT (NSGCT). The reason for this is that RPLND can accurately stage the retroperitoneum and thus positively identify those patients harboring metastases. In addition, patients with pathologic stage I disease are spared the toxicity and morbidity of any additional therapy because 90% or more experience long-term disease-free survival with surgery alone. Patients with pathologic stage II disease can learn more about the extent of their disease and make informed decisions regarding further therapy after RPLND. For patients in this group who harbor small-volume retroperitoneal disease (pN1), a properly performed RPLND can be curative in approximately 70% of men; thus chemotherapy also can be avoided in this setting (Richie and Kantoff, 1991; Donohue et al, 1993; Rabbani et al, 2001). Because the retroperitoneum is the most frequent site of chemoresistant malignant GCT and teratoma, both of these processes are minimized with RPLND (Baniel et al, 1995). Some groups advocate RPLND as the treatment of choice for all men with clinical stage I NSGCT with teratoma in the orchiectomy specimen given the increased propensity of harboring teratoma in the retroperitoneum (Sheinfeld et al, 2003). RPLND eliminates these chemoresistant elements and maximizes therapeutic efficacy. Traditionally RPLND for GCTs has been performed via an open transabdominal or thoracoabdominal approach. Over the past 2 decades minimally invasive approaches for the treatment of various malignancies have emerged and become popular. Since the early 1990s, retroperitoneal laparoscopic surgery has been utilized with proven benefits related to reducing perioperative morbidity, improving cosmesis, and shortening convalescence without compromising oncologic efficacy (Cadeddu et al, 1998; Allaf et al, 2004; Permpongkosol et al, 2005). Laparoscopic RPLND (L-RPLND) is a technically demanding procedure that is increasingly being performed by experienced laparoscopic surgeons aiming to minimize morbidity while duplicating the open technique. Given that untreated retroperitoneal disease and late relapses in the retroperitoneum are fatal and can be chemorefractory, it is of paramount importance that, just like in open RPLND, a complete “clean out” of lymph nodes is performed (Whitmore, 1979; Borge et al, 1988; Baniel et al, 1995; Carver et al, 2005). In an effort to decrease the morbidity associated with open RPLND, shortly after the introduction of laparoscopic renal surgery in 1991 several reports emerged documenting the feasibility of L-RPLND in the management of clinical stage I NSGCT (Rukstalis and Chodak, 1992; Stone et al, 1993; Klotz, 1994). Larger retrospective series followed suggesting decreased blood loss, shorter hospital stays, and faster return to normal activity when compared with open RPLND, with preservation of antegrade ejaculation in more than 95% of patients (Gerber et al, 1994; Janetschek et al, 1994, 1996). An early multi-institutional retrospective analysis demonstrated preservation of antegrade ejaculation in all patients, short hospital stays (<3 days), and return to normal activity at 2 to 3 weeks postoperatively (Gerber et al, 1994). The abbreviated convalescence allows patients who are candidates to receive chemotherapy with minimal delay. These attractive early results encouraged others to investigate L-RPLND as a viable treatment option for low-stage NSGCT. Of all laparoscopic applications to surgical urology, L-RPLND has raised the most controversy. This is due to the technical difficulty of RPLND in general, the limited number of cases, as well as lack of interest at traditional centers of excellence. Laparoscopy is an access technique with the internal procedure being performed the same as that with an open incision. As such, experience drives an equivalent dissection. In all early series and some contemporary studies, L-RPLND was used as a staging procedure (Bianchi et al, 1998; Janetschek et al, 2000). Patients not harboring occult metastases were identified and spared exposure to chemotherapy without undergoing open RPLND. In this form, L-RPLND was performed without retrocaval or retroaortic dissection and chemotherapy was given to all patients harboring metastatic disease (including patients with pN1 disease). The decision to omit dissection behind the great vessels was based on the belief of a lack of isolated positive lymph nodes in this area (Holtl et al, 2002). Within this paradigm, the procedure was routinely aborted if positive lymph nodes were encountered and chemotherapy was instituted in these cases (Bianchi et al, 1998; Nelson et al, 1999). In contemporary series this approach has been abandoned and L-RPLND has evolved into a therapeutic procedure duplicating the open approach in its intent (Allaf et al, 2005; Steiner et al, 2008). The use of restrictive template boundaries coupled with the universal use of chemotherapy in men harboring pathologic stage II disease generated criticism of published L-RPLND series. The controversy regarding the use of “staging” L-RPLND hinges on mapping studies demonstrating increased multifocality and contralateral disease in the presence of positive retroperitoneal nodes (Ray et al, 1974; Donohue et al, 1982; Weissbach and Boedefeld, 1987; Eggener et al, 2007). Critics argue that the liberal use of chemotherapy will not necessarily prevent relapses and compensate for incomplete resection. Currently at experienced centers an exact replication of the open template is performed on all patients with NSGCT undergoing L-RPLND with wide templates and complete excision of retroaortic and retrocaval tissue, thus rendering the procedure both a staging and a therapeutic operation. Some groups perform a bilateral dissection on all patients whereas others reserve it for patients with lymph node involvement (Allaf et al, 2005; Steiner et al, 2008). All patients considered candidates for L-RPLND must be fully informed of all treatment options, including open RPLND, chemotherapy, and surveillance. All potential complications, including bleeding requiring blood transfusion, injury to adjacent organs (liver, bowel, gallbladder, kidney, ureter, pancreas, major vascular structures), and orthopedic, neurologic, or pulmonary complications, as well as conversion to open surgery due to complications or incomplete resection, should be discussed (Allaf et al, 2005; Winfield, 1998). Patients interested in future fertility are educated regarding preoperative sperm banking. Some surgeons advocate a low-fat diet 1 to 2 weeks before surgery to reduce the risk of chylous ascites, but data regarding this practice are not definitive. Patients undergo a mechanical bowel preparation the afternoon before surgery and take only clear liquids until midnight to decompress the bowels. Preoperative antibiotics are given before surgery, and antiembolism devices are placed on the lower extremities to minimize deep vein thrombosis. Although some surgeons utilize an extraperitoneal approach (Hsu et al, 2003; Hara et al, 2004) most prefer a transperitoneal approach owing to the larger and more familiar working space. Additionally, a transperitoneal approach facilitates bilateral dissection when warranted by allowing access to all four quadrants. After general anesthesia is induced, an orogastric tube and Foley catheter are inserted. The patient may be placed in the modified flank position (45 degrees) with the side of dissection elevated, but the authors prefer the supine position because it makes transitioning to a bilateral dissection less cumbersome and does not require patient repositioning (Fig. 33–1). Great care is taken to pad all pressure points to minimize the risk of nerve injury or rhabdomyolysis because these surgeries may require a longer time than their open counterpart. The patient must be secured to the operating table because tilt is needed to use gravity to help shift the bowel out of the operative field. After intraperitoneal access is achieved (via a Veress needle or Hasson technique), four equally spaced 10/12-mm laparoscopic ports are placed in the midline beginning 1 cm below the xiphoid process (Fig. 33–2). As such, the umbilicus may not necessarily be incorporated as a port site. The large port size is essential to allow for the convenient introduction of larger (10/12-mm) instruments from varying angles. An additional 5-mm port may be placed in the midaxillary line midway between the iliac crest and ribs for additional retraction if needed. The bed is rotated maximally to allow optimal medialization of the bowel away from the operative field. The camera is moved to the second to the bottom trocar (trocar 3 in Fig. 33–2) to facilitate visualization during dissection of the spermatic cord stump. The peritoneum medial to the spermatic cord is incised, and the vas deferens is transected. The peritoneum is incised circumferentially around the inguinal ring (Fig. 33–3). With gentle traction on the cord, fibrous attachments and scar are incised until the suture on the spermatic cord is identified. The attachments are cut, and the cord is followed proximally along with surrounding nodal and fibroadipose tissue to the inferior vena cava (IVC). The ureter must be identified at all times to prevent inadvertent thermal injury. The spermatic vein and artery are both ligated proximally and transected. The specimen is placed in an endobag and dropped on the contralateral side of the abdomen.
Rationale and Evolution
Staging L-Rplnd and Controversy
Duplication of Open Rplnd
Surgical Technique
Preoperative Patient Preparation and Technical Considerations
Approach
Patient Positioning and Port Placement
Right-Sided Dissection
Spermatic Cord Dissection
Laparoscopic Retroperitoneal Lymphadenectomy for Testicular Tumors