Laparoscopic Pancreatic Surgery
Attila Nakeeb
 INDICATIONS/CONTRAINDICATIONS
INDICATIONS/CONTRAINDICATIONSOver the past decade significant advances have been made in the application of minimally invasive techniques to the management of both benign and malignant pancreatic disorders. Initially, laparoscopic pancreatic surgery was limited to diagnostic staging in patients with pancreatic cancer prior to resection. With recent advancements in laparoscopic instrumentation, an increasing number of surgeons are using laparoscopic techniques to resect benign and malignant lesions of the pancreas in carefully selected patients. Laparoscopic pancreaticoduodenectomy (PD), central pancreatectomy (CP), pancreatic enucleation (En), and distal pancreatectomy (DP) have all been described in the literature.
Potential advantages of laparoscopic surgery include decreased postoperative pain, decreased ileus, preserved immune function, decreased complication rates, shorter hospital stay and a quicker return to preoperative activity levels. While there have been no randomized prospective trials comparing laparoscopic to open pancreatic resections, several retrospective studies have shown laparoscopic resections to be associated with decreased intraoperative blood loss, lower overall complication rates, and a shorter hospital length of stay.
Laparoscopic pancreatic resections are complicated laparoscopic procedures and should be undertaken by surgeons with advanced laparoscopic skill sets. Surgeons should be comfortable with intracorporeal suturing, the use of endomechanical staplers, the use of laparoscopic ultrasound, and posses the ability to control intraoperative hemorrhage. In addition, surgeons should have experience with open pancreatic surgery in case the procedure must be converted to an open pancreatic resection.
Factors important in selecting appropriate patients for laparoscopic resections include the size of the lesion, location of the lesion within the pancreas (head/uncinate vs. body vs. tail), involvement of surrounding structures, and the suspected pathology of the lesion. Lesions that are potentially amenable to a laparoscopic pancreatic resection include benign or premalignant cystic neoplasms, small pancreatic endocrine neoplasms (neuroendocrine or islet cell tumors), pancreatic pseudocysts or isolated strictures of the pancreatic duct localized to the distal body and tail of the pancreas
(Table 4.1). While there are a small number of reports of laparoscopic pancreatic resections for adenocarcinoma of the pancreas, it is unclear if a laparoscopic pancreatic resection should be attempted in patients with malignant neoplasms of the pancreas.
(Table 4.1). While there are a small number of reports of laparoscopic pancreatic resections for adenocarcinoma of the pancreas, it is unclear if a laparoscopic pancreatic resection should be attempted in patients with malignant neoplasms of the pancreas.
TABLE 4.1 Indications for Laparoscopic Pancreatic Resection | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNINGImaging
A number of different imaging options are available to help the surgeon determine the appropriate surgical approach to diseases of the pancreas. These options include both noninvasive (e.g., transabdominal ultrasonography, computed tomography, and magnetic resonance imaging) and invasive modalities (e.g., endoscopic retrograde cholangiopancreatography [ERCP] and endoscopic ultrasonography [EUS]).
Transabdominal ultrasonography can identify changes associated with chronic pancreatitis, biliary and pancreatic duct dilation, and the presence of pseudocysts. In the setting of malignant disease, it may demonstrate dilated intrahepatic and extrahepatic bile ducts, liver metastases, pancreatic masses, ascites, and enlarged peripancreatic lymph nodes. A malignancy of the pancreas typically appears as a hypoechoic mass; ultrasonography reveals a pancreatic mass in 60% to 70% of pancreatic cancer patients.
Helical (spiral) CT is now the preferred noninvasive imaging test for pancreatic disease, having largely supplanted ultrasonography in this context. Helical CT scan delineate the anatomy of the pancreas and the surrounding organs in considerable detail. It can also easily define pancreatic calcifications, inflammation, necrosis, and masses. Pancreatic cancer usually appears as an area of pancreatic enlargement with a localized hypodense lesion. A triple phase intravenous contrast study is ideal for the assessment. Thin cuts are obtained through the pancreas and the liver during both the arterial phase and the venous phase after the injection of IV contrast material. Besides determining the primary tumor size, CT can also identify and evaluate invasion into local structures or metastatic disease.
In general, MRI has no significant advantages over CT: Its signal-to-noise ratio is low, it is prone to motion artifacts, it does not opacify the bowel, and it has low spatial resolution. Nevertheless, magnetic resonance cholangiopancreatography (MRCP) can be quite useful for defining the anatomy and pathology of the bile ducts and the pancreatic duct noninvasively. It can be especially helpful in cases where the ampulla of Vater is not accessible (as in patients who have previously undergone Roux-en-Y or Billroth II reconstructions).
ERCP allows direct imaging of the pancreatic and bile ducts and is the gold standard for diagnosing chronic pancreatitis. It also allows therapeutic stenting of biliary and pancreatic duct strictures. The sensitivity of ERCP for the diagnosis of pancreatic cancer
approaches 90%. The presence of a long, irregular stricture in an otherwise normal pancreatic duct is highly suggestive of a pancreatic malignancy. Often, the pancreatic duct is obstructed with no distal filling.
approaches 90%. The presence of a long, irregular stricture in an otherwise normal pancreatic duct is highly suggestive of a pancreatic malignancy. Often, the pancreatic duct is obstructed with no distal filling.
EUS now plays an important role in the evaluation of pancreatic diseases. It is a semi-invasive test that can be performed with a very low rate of complications (<0.1%). EUS can diagnose the most common causes of extrahepatic biliary obstruction (e.g., choledocholithiasis and pancreaticobiliary malignancies) with a degree of accuracy equaling or exceeding that of direct cholangiography or ERCP. It is the most sensitive modality for the diagnosis of pancreatic carcinoma. The particular strengths of EUS in the diagnosis of pancreatic cancer include its ability to (1) clarify small (<2 cm) lesions when CT findings are questionable or negative, (2) detect malignant lymphadenopathy, and (3) guide fine-needle aspiration (FNA) for definitive diagnosis and staging. The aspiration of cyst fluid and determination of cyst CEA levels and mucin can help differentiate serous from mucinous cysts.
Patient Preparation
Every patient considered for a pancreatic resection needs a full evaluation of cardiac, pulmonary and renal function. A full array of laboratory test must be obtained including a complete blood count, renal panel, and liver panel. A nutritional assessment needs to be made to make sure that the patient can undergo surgery safely, if patient has severe weight loss or has an albumin of <3 g/dL, strong consideration for supplemental nutrition is indicated. Serum tumor markers including CEA and CA19-9 are usually measured in patients with both solid and cystic tumors. If a neuroendocrine tumor is suspected by history (symptomatic), imaging (hypervascular on CT scan), or on preoperative biopsy than serum levels of chromogranin A, insulin, proinsulin, glucagon, gastrin, vasoactive intestinal peptide (VIP), or pancreatic peptide (PP) can be measured.
For lesions involving the body and tail of the pancreas the patient should receive vaccination against encapsulated organisms to prevent post splenectomy sepsis. These include Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae vaccines. The vaccines should be administered 1 or 2 weeks prior to the operation if possible.
 SURGICAL TECHNIQUE
SURGICAL TECHNIQUEInstrumentation
In addition to standard laparoscopic equipment available in most operating rooms, certain specialized equipment is necessary to safely carry out laparoscopic pancreatic surgery (Table 4.2). Intraoperative ultrasound is an invaluable tool during laparoscopic pancreatic resections. Ultrasound can be used to help localize lesions in the pancreas, define the relationship between the lesion and the pancreatic duct, assess for vascular invasion by the lesion, assess resection margins, and rule out metastatic disease.
TABLE 4.2 Equipment for Laparoscopic Pancreatic Resection | |
|---|---|
|
Positioning and Room Setup
Following endotracheal intubation and general anesthesia, an orogastric tube and foley catheter are placed. Sequential compression devices and/or subcutaneous heparin are used for deep venous thrombosis prophylaxis and a first generation cephalosporin is used for infectious prophylaxis.
For lesions in the head, uncinate, or neck of the pancreas the patient is positioned supine and for lesions located in the body and tail of the pancreas I prefer to position the patient in a semilateral (30 to 45 degrees) position with the left side up. The surgeon and the camera operator stand on the patient’s right side, while the first assistant and the scrub nurse stand on the patients left side. Video monitors are placed over both shoulders and the laparoscopic ultrasound monitor is placed on the patients left side near the video monitor.
Laparoscopic Distal Pancreatectomy with or without Splenectomy
Splenic Vessel Preserving Spleen Preserving Distal Pancreatectomy
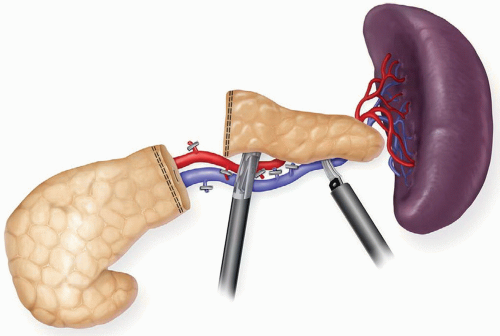
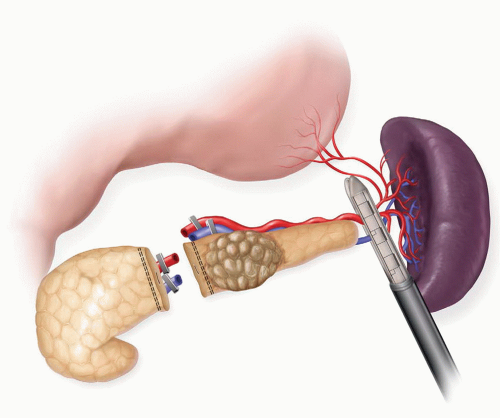
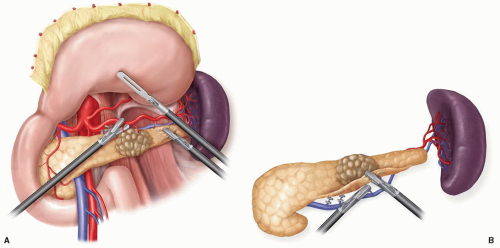
Laparoscopic distal pancreatectomy may be performed as a splenic preserving distal pancreatectomy (SPDP) or an en bloc distal pancreatectomy plus splenectomy. Two techniques for splenic preservation have been described. The first involves preservation of the splenic artery and vein and requires a very careful dissection and ligation of the small branches from the splenic artery and vein to the pancreas (Fig. 4.1). The second technique involves the division of the splenic artery and vein proximally; followed by a second division of the vessels as they emerge from the tail of the pancreas (Fig. 4.2). The spleen is vascularized by the short gastric vessels and the left gastroepiploic vessels. Attempts at splenic preservation are appropriate for benign cystic neoplasms and neuroendocrine tumors. Splenectomy is often necessary if the tail of the pancreas extends well into the splenic hilum or there is significant peripancreatic inflammation that makes dissection of the pancreas off of the splenic vessels hazardous. Splenectomy should be performed if the procedure is being done for malignancy or there is left-sided portal hypertension secondary to splenic vein thrombosis.
Splenic Vessel Preserving Spleen Preserving Distal Pancreatectomy
Port Placement
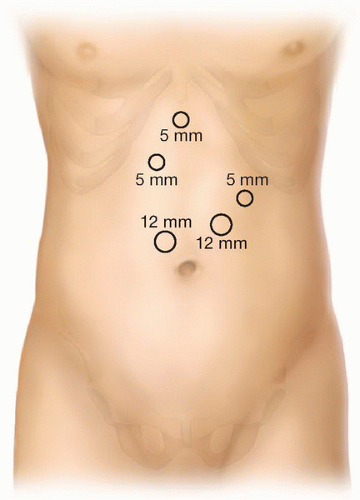
Access to the peritoneal cavity is achieved by either an open technique or via an Optiview technique. Five ports are placed (Fig. 4.3), and a 10 mm 30-degree laparoscope is used. As in all pancreatic procedures, the peritoneal surfaces, the omentum, the mesentery, and the viscera should all be carefully inspected to rule out metastatic disease. Intraoperative ultrasonography may be employed to evaluate the liver and locate the lesion in the pancreas.
Exposure of the Pancreas
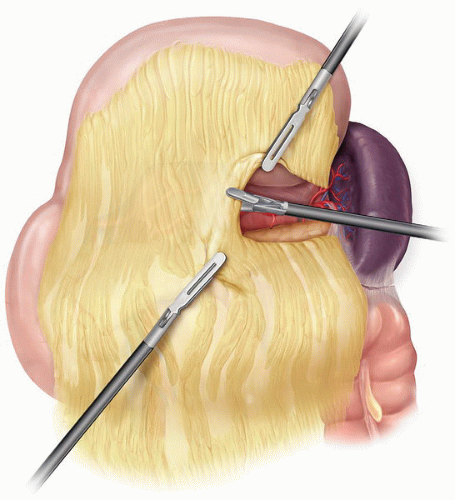
The body and tail of the pancreas are exposed by opening the lesser sac (Fig. 4.4). The gastrocolic omentum is divided and widely mobilized with an ultrasonic dissector, with
care taken to stay outside the gastroepiploic vessels. The short gastric vessels should not be divided if a splenic preserving procedure is being attempted. A retractor is advanced into the lesser sac through the subxiphoid port and used to elevate the stomach anteromedially. Alternatively, the stomach can be sutured to the anterior abdominal wall with a temporary suture to obtain exposure. The splenocolic ligament is divided, and the splenic flexure of the colon is reflected inferiorly.
care taken to stay outside the gastroepiploic vessels. The short gastric vessels should not be divided if a splenic preserving procedure is being attempted. A retractor is advanced into the lesser sac through the subxiphoid port and used to elevate the stomach anteromedially. Alternatively, the stomach can be sutured to the anterior abdominal wall with a temporary suture to obtain exposure. The splenocolic ligament is divided, and the splenic flexure of the colon is reflected inferiorly.
Mobilization of the Pancreas
After exposure of the pancreas, the peritoneum is incised along the inferior pancreatic border, and the pancreatic body is separated from the retroperitoneum by means of sharp and blunt dissection along its inferior border. Laparoscopic ultrasonography and direct visual inspection, combined with the findings from preoperative imaging, may be employed to determine the extent of the dissection. Initially, the dissection should be directed so that it is medial to the pancreatic lesion.
Isolation of the Splenic Vein
The pancreatic body is elevated by means of blunt and sharp dissection, after which the splenic vein should be easily identifiable (Fig. 4.5). Care must be exercised to prevent inadvertent injury to this vessel. Once the splenic vein has been identified, a careful circumferential dissection around the splenic vein is performed and a vessel loop can be placed around the vein for retraction.
Isolation of the Splenic Artery
The splenic artery can be identified from the under surface of the pancreas by retracting on the vessel loop around the splenic vein or it can be identified along the superior border of the pancreas anteriorly (Fig. 4.5). Once it is dissected circumferentially it is also controlled with a vessel loop. These precautionary measures allow quick control of bleeding should a vascular tear occur later in the procedure.
Parenchymal Transection
Once vascular control of the splenic artery and vein has been achieved, the pancreas is dissected off of the vessels in preparation for transaction of the gland. My preference
is to transect the pancreas with an ultrasonic scalpel. We have found the ultrasonic dissector to be hemostatic and to easily transect both firm and soft glands. It is important to move through the pancreas slowly and to start applying energy as the jaws are closing. If the pancreas is transected with an energy device I then treat the pancreatic remnant with a saline coupled radiofrequency sealing device (Salient Surgical Technologies) to seal the end of the remnant pancreas. Alternatively, an endoscopic stapler with or without staple line reinforcement can be placed across the body of the pancreas and used to divide the parenchyma (Fig. 4.6). If the pancreatic duct is visualized it is oversewn with a 4-0 absorbable suture.
is to transect the pancreas with an ultrasonic scalpel. We have found the ultrasonic dissector to be hemostatic and to easily transect both firm and soft glands. It is important to move through the pancreas slowly and to start applying energy as the jaws are closing. If the pancreas is transected with an energy device I then treat the pancreatic remnant with a saline coupled radiofrequency sealing device (Salient Surgical Technologies) to seal the end of the remnant pancreas. Alternatively, an endoscopic stapler with or without staple line reinforcement can be placed across the body of the pancreas and used to divide the parenchyma (Fig. 4.6). If the pancreatic duct is visualized it is oversewn with a 4-0 absorbable suture.
Medial to Lateral Dissection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree