Laparoscopic adrenalectomy has become an accepted method for removing benign lesions of the adrenal gland. There are few contraindications to the laparoscopic approach, and the transperitoneal and retroperitoneal techniques yield excellent results. Virtually all benign lesions and select malignant lesions can be removed laparoscopically. Laparoscopic adrenalectomy has been shown to be a safe and effective approach to many forms of adrenal pathologic conditions. It should be considered the standard of care in the management of benign lesions of the adrenal gland that require surgical removal.
Since originally reported by Gagner and colleagues in 1992, laparoscopic adrenalectomy has become an established procedure that has replaced the open approach in virtually all cases. Numerous studies have demonstrated the advantages of the laparoscopic approach, including decreased blood loss, less patient morbidity, shorter hospitalization, faster recovery, and cost-effectiveness . The indications for laparoscopic adrenalectomy have increased, whereas the absolute contraindications have decreased. As such, laparoscopic adrenalectomy has become a standard of care and the technique of choice for most benign adrenal lesions.
This article discusses the diagnosis and evaluation, indications, surgical technique, and results of laparoscopic adrenalectomy for benign and malignant conditions.
Diagnosis
In the past, most adrenal lesions were diagnosed secondary to clinical manifestations resulting from hormonally active adrenal tumors. Because of widespread use of ultrasound, CT scans, and MRI, however, most adrenal lesions are diagnosed incidentally. Figs. 1 and 2 demonstrate examples of adrenal lesions diagnosed on CT and MRI. The differential diagnosis of an incidental adrenal mass includes benign nonfunctioning adenoma, hormonally active cortical tumor, myelolipoma, pheochromocytoma, adrenocortical carcinoma, and metastatic lesion.
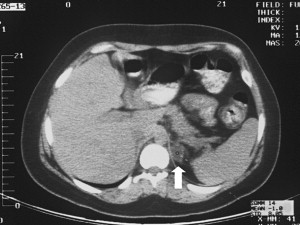
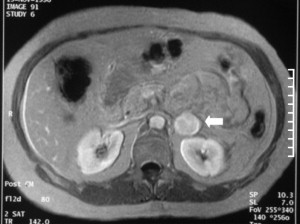
Endocrinologic evaluation
Hormonal evaluation is required in all patients who have adrenal lesions, regardless of size, to determine whether there is functional activity present. This evaluation is particularly important in patients who are undergoing adrenal surgery because of postoperative considerations regarding blood pressure control, electrolyte status, volume status, and other anesthesia considerations. Box 1 lists standard laboratory tests used for evaluating patients who have adrenal lesions. In general, most hormonally active adrenal tumors should be removed. On occasion, medical management of aldosteronomas may be achieved with spironolactone, particularly when patients are poor surgical candidates .
Cushing’s syndrome
24-hour urine cortisol
Plasma corticotropin and plasma cortisol
Low-dose dexamethasone suppression test
High-dose dexamethasone suppression test
Metapyrone stimulation test
Petrosal sinus corticotropin measurement
Hyperaldosteronism
Unprovoked hypokalemia
Plasma aldosterone level
Urinary aldosterone level
Aldosterone-to-renin ratio
Postural stimulation test
Adrenal vein sampling of aldosterone
Pheochromocytoma
Plasma catecholamines
Urine catecholamines and metanephrines
Clonidine suppression test
Adrenal vein sampling of catecholamines
Cushing’s syndrome
Cushing’s syndrome refers to the clinical condition that results from excess circulating glucocorticoids. There are several nonadrenal causes of Cushing’s syndrome, but the urologist most commonly encounters a patient who has an adrenal cortical tumor as the cause of Cushing’s syndrome. Features of Cushing’s syndrome are well recognized, including hypertension, truncal obesity, moon facies, easy bruising, and mood disorders. Diagnosis is made by collecting a 24-hour urinary cortisol measurement . The low-dose dexamethasone suppression test can be used to diagnose Cushing’s syndrome further if the urinary cortisol measurement is equivocal. An abdominal CT scan and MRI are used to identify adrenal adenomas or bilateral adrenal hyperplasia. Adrenal adenomas causing Cushing’s syndrome are amenable to laparoscopic adrenalectomy. Other than optimizing the patient’s general medical condition, there are no specific additional recommendations that are required in patients who have Cushing’s syndrome before undergoing laparoscopic adrenalectomy.
Aldosteronoma
Primary hyperaldosteronism (Conn’s syndrome) is the clinical syndrome that arises when elevated aldosterone levels result in increased total body sodium content and a decrease in potassium. It is a rare cause of hypertension, with other symptoms, including paresthesias, muscle weakness, and, on occasion, visual disturbances . Adrenal aldosteronomas as small as 1 cm can be sufficient to cause the symptoms. Typical laboratory findings include hypokalemia, hypernatremia, elevated plasma and urine aldosterone levels, an elevated serum aldosterone-to-renin ratio, and suppressed plasma renin activity . Adrenal vein sampling is rarely required.
Once the diagnosis is confirmed, medical control of hypertension and correction of hypokalemia should be instituted several weeks before adrenalectomy. Spironolactone, a competitive antagonist of the aldosterone receptor, is the most effective medication for management of hyperaldosteronism. Alternative medications include potassium-sparing diuretics, calcium channel blockers, and converting enzyme inhibitors. Unless they are poor candidates for surgery, adrenalectomy is recommended in all patients, with hypertension being improved or cured in more than 90% of patients after surgery .
Pheochromocytoma
Pheochromocytomas are tumors that release adrenal catecholamines, which can result in hypertension, tachycardia, and a host of clinical manifestations. Laboratory diagnosis is made by identifying elevated levels of catecholamines in the blood and urine. Radiographic diagnosis is made with CT or MRI, with MRI demonstrating a bright image on a T2-weighted study. Additionally, MIBG nuclear medicine scanning can help to confirm and localize pheochromocytomas .
Because of the unique manifestations of catecholamine excess, successful laparoscopic adrenalectomy for pheochromocytoma requires collaboration with the surgeon, endocrinologist, and anesthesiologist. Although pheochromocytoma was once considered a relative contraindication to laparoscopic surgery, laparoscopic adrenalectomy for pheochromocytomas has now been performed successfully and reported in several series . Preoperative medical preparation includes optimal control of blood pressure with alpha-blockade or calcium channel antagonists . Beta-blockers may be used to control reflex tachycardia after initiation of the alpha-blockade. During surgery, aggressive fluid expansion is necessary to increase circulating plasma volume and prevent postoperative hypotension. Close monitoring during surgery includes careful attention to blood pressure, central venous pressure, and urinary output.
Endocrinologic evaluation
Hormonal evaluation is required in all patients who have adrenal lesions, regardless of size, to determine whether there is functional activity present. This evaluation is particularly important in patients who are undergoing adrenal surgery because of postoperative considerations regarding blood pressure control, electrolyte status, volume status, and other anesthesia considerations. Box 1 lists standard laboratory tests used for evaluating patients who have adrenal lesions. In general, most hormonally active adrenal tumors should be removed. On occasion, medical management of aldosteronomas may be achieved with spironolactone, particularly when patients are poor surgical candidates .
Cushing’s syndrome
24-hour urine cortisol
Plasma corticotropin and plasma cortisol
Low-dose dexamethasone suppression test
High-dose dexamethasone suppression test
Metapyrone stimulation test
Petrosal sinus corticotropin measurement
Hyperaldosteronism
Unprovoked hypokalemia
Plasma aldosterone level
Urinary aldosterone level
Aldosterone-to-renin ratio
Postural stimulation test
Adrenal vein sampling of aldosterone
Pheochromocytoma
Plasma catecholamines
Urine catecholamines and metanephrines
Clonidine suppression test
Adrenal vein sampling of catecholamines
Cushing’s syndrome
Cushing’s syndrome refers to the clinical condition that results from excess circulating glucocorticoids. There are several nonadrenal causes of Cushing’s syndrome, but the urologist most commonly encounters a patient who has an adrenal cortical tumor as the cause of Cushing’s syndrome. Features of Cushing’s syndrome are well recognized, including hypertension, truncal obesity, moon facies, easy bruising, and mood disorders. Diagnosis is made by collecting a 24-hour urinary cortisol measurement . The low-dose dexamethasone suppression test can be used to diagnose Cushing’s syndrome further if the urinary cortisol measurement is equivocal. An abdominal CT scan and MRI are used to identify adrenal adenomas or bilateral adrenal hyperplasia. Adrenal adenomas causing Cushing’s syndrome are amenable to laparoscopic adrenalectomy. Other than optimizing the patient’s general medical condition, there are no specific additional recommendations that are required in patients who have Cushing’s syndrome before undergoing laparoscopic adrenalectomy.
Aldosteronoma
Primary hyperaldosteronism (Conn’s syndrome) is the clinical syndrome that arises when elevated aldosterone levels result in increased total body sodium content and a decrease in potassium. It is a rare cause of hypertension, with other symptoms, including paresthesias, muscle weakness, and, on occasion, visual disturbances . Adrenal aldosteronomas as small as 1 cm can be sufficient to cause the symptoms. Typical laboratory findings include hypokalemia, hypernatremia, elevated plasma and urine aldosterone levels, an elevated serum aldosterone-to-renin ratio, and suppressed plasma renin activity . Adrenal vein sampling is rarely required.
Once the diagnosis is confirmed, medical control of hypertension and correction of hypokalemia should be instituted several weeks before adrenalectomy. Spironolactone, a competitive antagonist of the aldosterone receptor, is the most effective medication for management of hyperaldosteronism. Alternative medications include potassium-sparing diuretics, calcium channel blockers, and converting enzyme inhibitors. Unless they are poor candidates for surgery, adrenalectomy is recommended in all patients, with hypertension being improved or cured in more than 90% of patients after surgery .
Pheochromocytoma
Pheochromocytomas are tumors that release adrenal catecholamines, which can result in hypertension, tachycardia, and a host of clinical manifestations. Laboratory diagnosis is made by identifying elevated levels of catecholamines in the blood and urine. Radiographic diagnosis is made with CT or MRI, with MRI demonstrating a bright image on a T2-weighted study. Additionally, MIBG nuclear medicine scanning can help to confirm and localize pheochromocytomas .
Because of the unique manifestations of catecholamine excess, successful laparoscopic adrenalectomy for pheochromocytoma requires collaboration with the surgeon, endocrinologist, and anesthesiologist. Although pheochromocytoma was once considered a relative contraindication to laparoscopic surgery, laparoscopic adrenalectomy for pheochromocytomas has now been performed successfully and reported in several series . Preoperative medical preparation includes optimal control of blood pressure with alpha-blockade or calcium channel antagonists . Beta-blockers may be used to control reflex tachycardia after initiation of the alpha-blockade. During surgery, aggressive fluid expansion is necessary to increase circulating plasma volume and prevent postoperative hypotension. Close monitoring during surgery includes careful attention to blood pressure, central venous pressure, and urinary output.
Indications for laparoscopic adrenalectomy
The indications for laparoscopic adrenalectomy have expanded as more surgeons have become proficient with the technique and the advantages of this approach have become apparent. In many centers, laparoscopic adrenalectomy has become the surgical procedure of choice for the management of functional tumors less than 6 cm in size. The current indications for performing laparoscopic adrenalectomy are listed in Box 2 .
Hormonally active adrenal tumor
Aldosteronoma
Pheochromocytoma
Cortisol-producing adrenal tumor
Nonfunctioning adrenal lesion greater than 5 cm in size
Nonfunctioning adrenal lesion with progressive growth
Solitary adrenal metastasis with negative metastatic survey
In general, most hormonally active tumors should be removed unless patients are poor candidates for surgery. Conversely, hormonally inactive tumors are managed according to size. Tumors less than 3 cm in size are almost always benign adenomas and generally require no further treatment unless clinical signs of hormonal activity develop . Most adrenocortical carcinomas are larger than 6 cm in size . Because CT is thought to underestimate the size of such lesions by as much as 1 cm , surgery is recommended for lesions greater than 5 cm in size. Hormonally inactive lesions between 3 and 6 cm in size can be followed with serial imaging.
There are few absolute contraindications to laparoscopic adrenalectomy. In cases of suspected primary adrenal carcinoma, particularly with extension into surrounding organs, open surgery should be performed. Given the aggressive nature of the disease, the open approach allows for en bloc resection and potential removal of surrounding organs . Other absolute contraindications to laparoscopic adrenalectomy include uncorrectable coagulopathy, severe cardiopulmonary disease, and uncontrolled pheochromocytoma.
Relative contraindications to laparoscopic adrenalectomy include extensive previous surgery and pregnancy. Caution should be used when doing laparoscopic adrenalectomy on lesions greater than 12 cm because of the increased risk for hemorrhage and injury to surrounding viscera. With increasing experience in performing laparoscopic adrenalectomy, relative contraindications have decreased.
Occasionally, the urologist encounters a patient who is suspected of having a solitary metastatic lesion to the adrenal gland. If the lesion is less than 6 cm in size and not obviously adherent to surrounding viscera, a laparoscopic approach is possible .
Surgical technique
Preoperative patient preparation and evaluation
Careful preoperative control and management of hormonally active tumors is necessary before performing laparoscopic adrenalectomy. Collaboration with an endocrinologist and anesthesiologist experienced with adrenal disorders is helpful. The urologist should have an understanding of the physiology of adrenal disorders to manage patients appropriately in the peri- and postoperative periods with regard to fluid management, electrolyte abnormalities, and blood pressure control. Hormonally functional tumors must be adequately evaluated, and appropriate preoperative interventions must be initiated in concert with an endocrinologist.
Before surgery, all patients should receive a mechanical bowel preparation. Clear liquids should be started the day before surgery. A broad-spectrum antibiotic should be administered on call to the operating room. Standard precautions for deep venous thrombosis prophylaxis, such as stockings and pneumatic compression devices, should be used.
Approach
There are several laparoscopic approaches to the adrenal gland. Commonly used approaches to the adrenal gland include the transperitoneal approach and the posterior and lateral retroperitoneal approaches. A transthoracic approach has been described for patients who have undergone extensive previous transperitoneal and retroperitoneal surgery . Surgeon preference and experience seem to be the most important factors in determining the approach. Although each approach has purported advantages and disadvantages, there is no evidence that one is superior .
Transperitoneal approach for right adrenalectomy
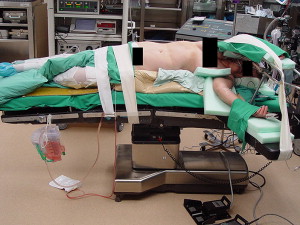
The patient is positioned on a beanbag with the right side elevated at 45° to 70° with the table slightly flexed at the level of the umbilicus. Fig. 3 shows the general modified flank position used for laparoscopic adrenalectomy. Next, the patient should be secured with tape to allow the table to be tilted side to side to facilitate exposure. A catheter is placed to drain the bladder, and an orogastric tube is placed to decompress the stomach.
After pneumoperitoneum is achieved, four subcostal ports are placed below the costal margin ( Fig. 4 ). Mobilization and retraction of the liver are necessary to expose the right adrenal gland. It is generally not necessary to mobilize the ascending colon and hepatic flexure fully. Incision of the posterior peritoneum and extension through the triangular ligaments of the liver allow for upward and medial retraction of the liver ( Fig. 5 ).
Full mobilization of the duodenum is often not necessary, because the inferior vena cava (IVC) is often identified once there is adequate liver mobilization. Further dissection of the IVC allows for visualization of the right adrenal vein. Extreme caution should be used when dissecting out the right adrenal vein because it is short and sometimes broad. Hemorrhage resulting from avulsion or injury to the right adrenal vein is extremely difficult to control because of its direct drainage into the IVC. The adrenal vein should be carefully divided between standard clips ( Fig. 6 ).









