Stress urinary incontinence and pelvic organ prolapse are prevalent conditions that can have detrimental effects on a woman’s quality of life. Surgically, this has often been approached by means of a transvaginal route. With recent advances in laparoscopic and robotic instrumentation and operating systems, there is increasing interest in minimally invasive techniques for correction of pelvic organ prolapse. In this article, the authors briefly describe the laparoscopic and robotic approaches in terms of surgical techniques, operative anatomy, and results published in the literature.
Disorders of the female pelvic floor, specifically stress urinary incontinence (SUI) and pelvic organ prolapse (POP), are prevalent among women of all ages. Numerous challenges await those who evaluate and treat these particular disorders, however. First, the risk factors for prolapse and incontinence are poorly understood, making it difficult to implement preventive measures. Second, there is no clear definition or agreement on what defines clinically significant prolapse. Objective measures of treatment are variable, and the definitions of success or failure of treatment options are vague. It has also been well established that discrepancies between subjective and objective outcome measures are not uncommon. Finally, we are hindered by the fact that much of the available literature is limited in length of follow-up and cohort size. These are important issues to recognize as we review the current literature.
The estimated lifetime risk for undergoing corrective surgery for POP is 11% . This estimate is based on hospital admission and surgical codes data and likely underestimates the overall prevalence of SUI and POP in the general population. Two studies examined data on POP from the large multi-institutional Women’s Health Initiative Hormone Replacement Therapy Clinical Trial. Hendrix and colleagues presented the baseline data from the 27,342 patients enrolled. On visual inspection alone during the Valsalva maneuver, 41.1% of patients with an intact uterus had some degree of descent versus 38% of patients who had undergone a hysterectomy. In an ancillary study, Nygaard and colleagues examined the rate of prolapse in women with an intact uterus. The mean age of the 270 participants was 68.3 years, and 65.5% were found to have stage 2 (leading edge within 1 cm proximal to hymen) or greater prolapse based on the standardized pelvic organ position quantification description (POP-Q ) examination. If the definition of prolapse was further narrowed to the leading edge at or beyond the hymen, 25.2% were defined as having prolapse.
In the past 10 years there has been significant evolution in the treatment of SUI and POP. The laparoscopic retropubic urethropexy was first introduced in 1991 but was abandoned because of several small studies reporting lower cure rates. The next major stride in minimally invasive treatment of SUI occurred in 1996 with the introduction of the tension-free vaginal tape (TVT) . This sling has been a significant impetus for the development of the vaginal mesh “kits” that are now available for treatment of SUI and POP. Attributable in great part to the simplicity of these techniques, the number of pelvic reconstructive cases performed per annum has increased significantly over the past decade.
In an effort to incorporate minimally invasive techniques in POP treatment, laparoscopic and robotic approaches have been applied to several transabdominal surgical techniques. The often-cited advantages of laparoscopic and robotic surgery include improved visualization because of magnification and insufflation, better cosmesis and hemostasis, shorter hospital stay and less postoperative pain with an overall more rapid recovery relative to corresponding open approaches. The disadvantages of minimally invasive techniques are the long “learning curve,” the potentially increased difficulty of the retroperitoneal dissection, and the potential increase in hospital costs. It is likely that with the increased application of robotics and laparoscopy, the learning curve is eventually likely to become a less significant barrier.
Only recently have well-designed trials with longer term and appropriate minimum follow-up (1–2 years) begun to appear in the literature. The aim of this article is to review the laparoscopic and robotic techniques that have been described for treatment of POP and SUI and to present the longer term results available to date. Although considered by many to be “minimally invasive,” the transvaginal techniques are not covered in this article.
Operative indications and anatomy
The indications for a laparoscopic or robotic approach to surgery for POP or SUI are identical to those for the open abdominal and vaginal routes. Repairs are reserved for those patients who are symptomatic from the prolapse. Symptoms may vary from patient to patient. The obvious bulge protruding from the vagina creating significant discomfort for the patient provides a clear indication for repair. More subtle findings of lower degrees of prolapse with variable associated voiding symptoms may require more contemplation.
The decision between a vaginal, abdominal, or laparoscopic or robotic approach depends on surgeon experience and patient preference. Other factors, such as prior prolapse repairs or anti-incontinence surgery, prior abdominal procedures, vaginal caliber and length in addition to age, weight, comorbidities, and ability to tolerate general anesthetic, are all important considerations for a laparoscopic or robotic approach.
The endopelvic fascia contributes to the integrity of the vaginal wall—anteriorly, the endopelvic fascia is referred to as the pubocervical fascia, and, posteriorly, the rectovaginal fascia. DeLancey described three levels of support to the vagina and pelvic structures based on suspension, attachment, and fusion of the pelvic tissues at the corresponding levels ( Fig. 1 ; Table 1 ). POP represents a break in the continuity of the endopelvic fascia or loss of the corresponding suspension, attachment, or fusion. Pelvic reconstructive surgery aims to re-establish and correct the defects to restore normal anatomy and visceral (bowel and bladder) and sexual function.
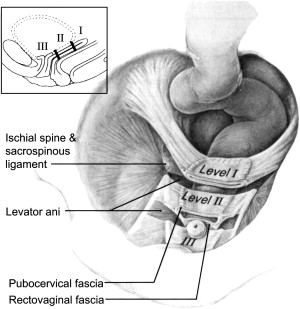
| Technique | Support mechanism | Supported structure | Result of support failure | |
|---|---|---|---|---|
| Level I | Suspension | Cardinal/uterosacral complex |
|
|
| Level II | Attachment | Lateral attachment of the pubocervical and rectovaginal endopelvic fascia to the ATFP |
|
|
| Level III | Fusion | Fusion to the perineal body | Distal one fourth of the vagina |
|
With any surgical procedure (open, laparoscopic, or robotic), there are important anatomic landmarks with which the surgeon must be familiar. Pertinent landmarks are discussed as the various approaches to pelvic floor reconstruction are reviewed.
Laparoscopic surgery for stress urinary incontinence: procedure and results
In 2004, the Third International Consultation on Incontinence reported that level 1 evidence to support the use of open Burch colpopexy for the treatment of SUI exists; however, data for laparoscopic colposuspension were insufficient, and no recommendations could be made regarding its use . Conversely, the 2005 Cochrane review reported that laparoscopic colposuspension provided benefits, such as more rapid recovery, but that long-term efficacy is unknown .
In 2006, two randomized trials of open versus laparoscopic Burch colposuspension with a minimum follow-up of 24 months suggested similar outcomes between the two procedures. Kitchener and colleagues reported a 2-year objective cure rate (<1 g on pad test) of 79.7% in the laparoscopic group versus 70.1% for the open group. Similar subjective data (never or <1 incontinent episode per month) of 55.4% versus 53.1%, respectively, were also demonstrated. Carey and colleagues reported subjective (no incontinence) results at 24 months of follow-up for all subjects of 63% and 70% for laparoscopic versus open approaches, respectively. With an intent-to-treat analysis, the success rates decreased to 61% and 50%, respectively, but this did not represent a statistically significantly difference. Neither study commented on the development of POP after surgery.
These data suggest that when performed in a fashion similar to the open procedure, the laparoscopic Burch colposuspension may be comparable to open surgery. Together with the data supporting the use of the laparoscopic Burch colposuspension at the time of sacrocolpopexy for patients without symptoms of SUI , there may still be a role for the laparoscopic Burch colposuspension in conjunction with other laparoscopic or robotic procedures.
Laparoscopic surgery for stress urinary incontinence: procedure and results
In 2004, the Third International Consultation on Incontinence reported that level 1 evidence to support the use of open Burch colpopexy for the treatment of SUI exists; however, data for laparoscopic colposuspension were insufficient, and no recommendations could be made regarding its use . Conversely, the 2005 Cochrane review reported that laparoscopic colposuspension provided benefits, such as more rapid recovery, but that long-term efficacy is unknown .
In 2006, two randomized trials of open versus laparoscopic Burch colposuspension with a minimum follow-up of 24 months suggested similar outcomes between the two procedures. Kitchener and colleagues reported a 2-year objective cure rate (<1 g on pad test) of 79.7% in the laparoscopic group versus 70.1% for the open group. Similar subjective data (never or <1 incontinent episode per month) of 55.4% versus 53.1%, respectively, were also demonstrated. Carey and colleagues reported subjective (no incontinence) results at 24 months of follow-up for all subjects of 63% and 70% for laparoscopic versus open approaches, respectively. With an intent-to-treat analysis, the success rates decreased to 61% and 50%, respectively, but this did not represent a statistically significantly difference. Neither study commented on the development of POP after surgery.
These data suggest that when performed in a fashion similar to the open procedure, the laparoscopic Burch colposuspension may be comparable to open surgery. Together with the data supporting the use of the laparoscopic Burch colposuspension at the time of sacrocolpopexy for patients without symptoms of SUI , there may still be a role for the laparoscopic Burch colposuspension in conjunction with other laparoscopic or robotic procedures.
Laparoscopic and robotic procedures for treatment of pelvic organ prolapse: anatomy and procedures
Anterior prolapse
The fact that a single surgical technique has not been readily accepted for treating cystoceles suggests a complexity of the condition beyond current understanding. Anterior colporrhaphy has long been the primary approach to correction of cystoceles despite reported failure rates of 40% to 70% . This emphasizes the fact that several different defects can result in a cystocele, and, accordingly, the defect specific to a given patient needs to be identified and corrected.
In 1909, White described the lateral detachment of the endopelvic fascia from the arcus tendineus fasciae pelvis (ATFP) as a cause for anterior compartment prolapse and a transvaginal approach for repair. A corresponding abdominal paravaginal repair was described in 1976 and has been replicated laparoscopically .
The anatomic landmarks for a paravaginal repair are similar to those used for a Burch retropubic urethropexy. The space of Retzius is developed to identify the lateral detachment of the endopelvic fascia from the ATFP. Key structures include Cooper’s ligaments, accessory or aberrant obturator veins, obturator neurovascular bundles that lie 3 to 4 cm anterior to the ATFP, the bladder neck, the ATFP itself, and the arcus tendineus levator ani.
In a laparoscopic transperitoneal approach, the bladder must first be mobilized. After filling the bladder for identification, a transverse incision 2 cm cephalad to the bladder reflection is made between the medial umbilical folds. Dissection is performed through the loose areolar tissue in an infralateral direction, moving toward the posterior-superior aspect of the pubic symphysis until Cooper’s ligaments, the bladder neck, the obturator internus muscle and foramen, and the ATFP are identified. A vaginal manipulator (finger) helps to identify the torn edges of the pubocervical fascia from the ATFP. This paravaginal defect is closed with a series of nonabsorbable sutures starting distally, alternating sides. The most distal sutures (generally three sutures) incorporate vaginal tissue and the obturator internus and iliopectineal ligaments, whereas the most proximal suture only incorporates vaginal tissue and the iliopectineal ligament. The sutures are then tied without tension. If there is concern that significant urethral hypermobility is contributing to the SUI, the sutures may be tied more tightly . Further level I defects require correction with hysteropexy or colpopexy. Cystoscopy is performed to confirm ureteral patency.
Posterior prolapse
Most surgeons prefer a transvaginal route for rectocele repair ; however, a laparoscopic approach has been described. The important landmarks for the abdominal approach include the rectovaginal septum, its lateral attachments to the medial aspect of the levator ani muscles, and the perineal body. During the procedure, the rectovaginal space is developed to the level of the rectovaginal septum. The perineal body is secured to the rectovaginal septum, and the rectovaginal fascial defects are closed with nonabsorbable sutures. In addition, the iliococcygeus and rectovaginal fascia can be reapproximated, and a levator ani placation can be performed if needed.
Apical prolapse
Identification and treatment of apical descent is fundamental to the success of pelvic floor reconstruction. Apical repair involves obliteration of the defect responsible for re-establishment of apical support.
Culdoplasty
Laparoscopic Moschowitz and Halban procedures are performed in a manner identical to the open abdominal repairs using nonabsorbable sutures to obliterate the cul-de-sac. In the Moschowitz procedure, the suture is placed in a purse-string fashion circumferentially around the cul-de-sac, whereas the Halban procedure uses sutures placed longitudinally incorporating the sigmoid serosa, the peritoneum of the cul-de-sac, and the posterior vagina. Cystoscopy should be performed after intravenous administration of indigo carmine or methylene blue to confirm ureteral integrity, keeping in mind that the risk for ureteral kinking is reportedly greater with the Moschowitz procedure . Cadeddu and colleagues described a modified Moschowitz procedure in which the posterior vaginal fascia is reapproximated with the anterior wall of the rectum.
Enterocele excision and closure
During a laparoscopic enterocele repair, the enterocele sac is dissected laparoscopically or vaginally with identification of defects of the endopelvic fascia, pubocervical fascia, and rectovaginal fascia. For large enteroceles, a transvaginal approach is used to excise the redundant peritoneum and vagina, with care taken to avoid foreshortening or narrowing of the vagina. The vaginal apex and rectum can be delineated during laparoscopic dissection by intravaginal placement of an obturator, sponge-stick, or equivalent vaginal manipulator. A nonabsorbable suture placed in an interrupted fashion is then used to reapproximate the pubocervical and rectovaginal fascial edges until the defect is closed. Koninckx and colleagues described vaporization of the enterocele sac with the carbon dioxide laser. Enterocele closure and excision performed concomitantly with uterosacral ligament suspension re-establishes support of the vaginal apex.
Uterosacral ligament suspension
The uterosacral ligaments have been used as supporting structures to re-establish level I support to the vaginal apex or uterus. Important anatomic landmarks in the laparoscopic uterosacral ligament vaginal vault suspension (LUSVS) or laparoscopic uterosacral ligament uterine vault suspension suspension (LUSUS) include the pubocervical and rectovaginal fascias, the uterosacral ligament, and the ureters. The ureters are in close proximity (1–1.5 cm lateral) to the uterosacral ligament as it courses beneath the uterine artery.
For the LUSVS, the uterosacral ligament at the proximal portion of its break is sutured with a nonabsorbable suture to the vaginal apex. The apical stitch is placed through the full thickness of the uterosacral and cardinal ligament complex and the rectovaginal fascia, excluding the vaginal epithelium. Additional sutures are placed more proximally on the uterosacral ligaments for support of the rectovaginal fascia. If a concomitant enterocele repair is planned, the uterosacral ligaments are identified and tagged before dissection of the posterior vagina . Ross described an apical repair that brought the rectovaginal septum and uterosacral and cardinal ligaments together with the use of purse-string sutures and plication of the uterosacral ligaments. In that procedure, the peritoneum is dissected free from the vaginal apex and pubocervical fascia. A series of nonabsorbable sutures are placed in a purse-string fashion incorporating the left and right uterosacral and cardinal ligaments, the rectovaginal septum, and the posterior vaginal wall. The first sutures are placed in the uterosacral ligament 3 to 4 cm proximal to the vaginal apex; subsequent sutures are placed until the vaginal apex is reached, with the final suture incorporating the pubocervical fascia.
Several researchers have described the LUSUS with minor differences in techniques. The basic principle involves placement of a nonabsorbable suture full thickness through the uterosacral ligament at the level of the ischial spine and then again at its insertion at the lower uterine segment to create shortening of the ligament .
Sacrocolpopexy
( Movie 1: Robotic assisted laparoscopic sacrocolpopexy ∗
∗ Videos for this article can be accessed by visiting www.urologic.theclinics.com . In the online table of contents for this issue, click on “add-ons.”
) Laparoscopic and robotic sacrocolpopexy (L/RSCP) procedures have been described with the goal of replicating the open surgical technique. Important anatomic landmarks at the sacrum include the middle sacral artery and vein and the anterior longitudinal ligament on the sacral promontory. The aortic bifurcation and vena cava lie superiorly at L4 to L5. Along the lateral margins of the presacral space are the iliac vessels and ureters bilaterally and the sigmoid colon, which is reflected to the left.Final port placement configuration between the laparoscopic and robotic approaches is similar. For the laparoscopic approach, primary ports include an intraumbilical port and then a 10- to 12-mm port in each lower quadrant. One to two ancillary 5-mm ports may be placed at the level of the umbilicus lateral to the rectus muscle. For the robotic approach, the primary 12-mm port is placed at the inferior umbilical crease and two lateral 8-mm ports are then placed just below the level of the umbilicus and lateral to the rectus muscle. Two additional assistant ports are then placed in the lower quadrants.
Once port placement is complete and the robot, when employed, is docked, a vaginal obturator is used to delineate the vaginal apex. The peritoneum overlying the apex is dissected off the vagina, and the incision of the posterior peritoneum is extended cephalad toward the sacral promontory.
Exposure of the presacral space and sacral promontory can be facilitated by “airplaning” the patient to the left and using a “snake” retractor. For improved exposure, retraction of the sigmoid can be facilitated using a figure-of-eight silk suture placed through the tenia coli and exiting percutaneously. The incision in the peritoneum overlying the sacral promontory is continued longitudinally extending through the cul-de-sac, and the presacral fat is cleared to expose the sacral periosteum. Hemostasis can be achieved by coagulation or clip placement. A Y-shaped synthetic mesh or biologic graft is secured to the vagina with nonabsorbable sutures placed through the full thickness of the vaginal wall. The mesh is secured to the sacral promontory with two nonabsorbable sutures preplaced into the anterior spinous ligament or by means of sutures attached to titanium bone anchors. The mesh should be placed without tension to support the vagina in an anatomic position. Redundant mesh is excised, and the posterior peritoneum is reapproximated over the mesh. If the mesh cannot be completely retroperitonealized, sigmoid epiploic fat can be used to cover it.
In the presence of a uterus, sacrohysteropexy can be performed for uterine preservation. This involves dissecting the rectovaginal space for approximately one third of the length of the posterior vaginal wall. The mesh or biologic graft is secured in several rows along the rectovaginal fascia and posterior cervix to the level of the internal os. Peritoneal closure is then performed.
Surgeon preference and anatomic detail should dictate the use of concomitant procedures. In the presence of a deep cul-de-sac, a Halban or Moschcowitz “culdeplasty” may be used. Urethral hypermobility is addressed with laparoscopic Burch or paravaginal repair. In the presence of rectal prolapse, rectopexy with or without the assistance of a colorectal surgeon can be performed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







