Chapter 17 Lactation and Galactorrhea
PROLACTIN
Prolactin is a 198-amino acid polypeptide hormone secreted in pulsatile fashion from the anterior pituitary (adenohypophysis) by specific cells referred to as lactotrophs.1 These cells have a common origin with the growth hormone-secreting cells (i.e., somatotrophs). Both prolactin and growth hormone are classified as somatomammotropic hormones based on their structural similarities. Due to its structural similarity to growth hormone, prolactin was the last pituitary hormone to be isolated.
Prolactin has a short half-life of only 20 minutes and exists in heterogeneous forms, including big prolactin, a dimeric glycosylated form, and big big prolactin.2,3 Secretion has a circadian rhythm, with a higher average level of serum prolactin during sleep, especially during the rapid eye movement phase.
The prolactin receptor is a member of the cytokine receptor family. It is a single transmembrane polypeptide found in multiple tissues in addition to the mammary glands, including the liver, adrenal glands, lungs, testes, and ovaries.4 The effects of prolactin in many of these tissues remains unknown.5
Neuroendocrinology of Prolactin Secretion
The control of prolactin secretion by the anterior pituitary lies within the hypothalamus and is predominantly inhibitory. However, the hypothalamus releases both prolactin-inhibiting factors (PIF) and prolactin-releasing factors (PRF) that modulate the secretion of prolactin.6
Prolactin-inhibiting factors are released by the hypothalamus into the hypothalamo-hypophyseal portal system. Although other compounds have been shown to have inhibitory activity, dopamine appears to be the most important PIF.7 Interruption of the tuberoinfundibular tract and blocking dopamine receptors with “gene knockout” techniques both result in high prolactin levels.8 Gonadotropin-associated peptide and γ-aminobutyric acid are also PIFs.9
Prolactin secretion is regulated by a negative feedback loop wherein prolactin, acting via prolactin receptors in the median eminence, stimulates dopamine secretion.10 Dopamine acts via the adenylyl cyclase pathway to reduce prolactin secretion from the pituitary lactotrophs. The predominant dopamine receptor in the adenohypophysis is D2 (DRD2). Binding of dopamine to this receptor decreases cellular adenylyl cyclase activity and cyclic adenosine monophosphate.
Although the major control mechanism for prolactin is inhibitory, the hypothalamus also releases PRFs, which stimulate lactotrophs to secrete prolactin (Table 17-1). The primary physiologic PRF appears to be vasoactive intestinal peptide. However, there appear to be two more clinically relevant PRFs.11,12 The ability of thyrotropin-releasing hormone (TRH) to act as a PRF is believed to be the basis of the association between hypothyroidism and hyperprolactinemia, a condition that resolves with thyroid hormone replacement. Serotonin appears to be another PRF; use of any of the various selective serotonin reuptake inhibitors (SSRIs) is commonly associated with hyperprolactinemia.
Table 17-1 Prolactin Releasing Factors
| Thyrotropin-releasing hormone |
| Serotonin |
| Vasoactive intestinal peptide |
| Opioids |
| Growth hormone-releasing hormone |
| Gonadotropin-releasing hormone |
LACTATION
Breast Development
Prenatal
Normal development in utero requires fetal exposure to many hormones, including estrogens, progesterone, prolactin, insulin, cortisol, thyroxine, and growth hormone (Table 17-2). The endocrine system is essential for the proper development and function of the mammary glands.13,14 Gene knockout studies have demonstrated the importance of each of these hormones in fetal mammary gland development.15. These hormones work through normal hormone–receptor interactions using local growth factors in many cases.16,17
Table 17-2 Hormones Required for Normal Mammary Development
| Estrogen |
| Progesterone |
| Prolactin |
| Cortisol |
| Insulin |
| Thyroxine |
| Growth hormone |
Puberty
At puberty, estradiol is the major influence on breast development, and the breast contains both α and β estrogen receptors. However, multiple hormones are necessary for normal development, including all the hormones necessary for normal in utero development (see Table 17-2).
Under the influence of rising estradiol levels at puberty, the breast increases in size and the areola gains pigmentation.18 Most breast development at puberty is adipocyte differentiation under the influence of estrogens. Estrogens stimulate the lactiferous ducts to branch extensively. The terminal alveoli unit, however, is rudimentary.
The Mature Breast
Anatomy
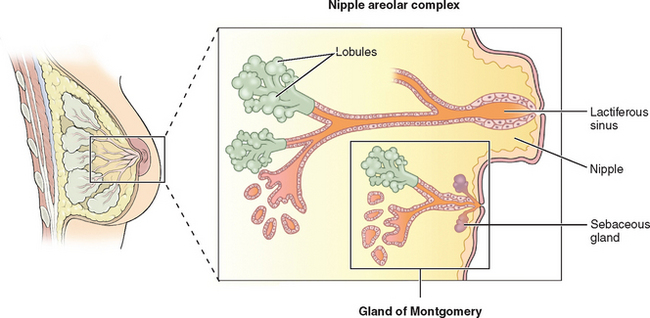
The breasts consist of glands, fat, and connective tissue. The basic glandular unit consists of ducts and secretory lobules (Fig. 17-1). Twenty or more lactiferous ducts converge on the nipple. Each large duct is connected to smaller branching ducts and eventually lobules. The ducts are lined by a cuboidal or columnar epithelium, at the base of which are myoepithelial cells. Each lobule contains alveoli, milk glands lined by epithelium.19,20 In a nonactive state, the ductules often have only rudimentary terminal alveoli that secrete milk.
Pregnancy
Breast development and lactogenesis during pregnancy occurs under the influence of multiple hormones, most notably prolactin, estrogen, and progesterone. Prolactin is secreted in large amounts by the decidua, and levels will be 5 to 10 times normal by the end of the first trimester.21 Under the influence of increased estrogen, the pituitary lactotrophs undergo hyperplasia. After delivery, prolactin declines to a normal baseline by 6 weeks postpartum and then pulses with breastfeeding.
Lactogenesis
Final differentiation of the breast into a lactating organ occurs during pregnancy. Lactogenesis is a three-stage process whereby mammary glands develop the ability to produce and secrete milk.22 Starting in early pregnancy and ending within a week of parturition, the mammary glands are hormonally transformed from an undifferentiated state to a fully differentiated state with an established supply of mature milk.
Stage I Lactogenesis
During this stage, mammary glands become competent to secrete milk. This stage begins early in gestation and is nearly complete by midpregnancy. As a result of rising levels of estrogen, progesterone, prolactin, and human placental lactogen, the terminal duct lobular units expand and secretory cells begin to differentiate. Prolactin induces the transcription of β-casein and lactalbumin genes.23
Stage II Lactogenesis
This stage begins at the time of birth and ends with the establishment of an appropriate milk supply. Delivery of the placenta results in a sudden drop in circulating progesterone levels. At the same time, both glucocorticoid and prolactin levels increase. This leads to increased synthesis of lactose. Lactose draws water by osmosis into the secretory vesicles. At the same time, synthesis of other milk components (e.g., nutrients and minerals) is increased under the influence of maternal insulin, growth hormone, cortisol, and parathyroid hormone. Secretion of the alveolar cells first leads to colostrum formation. Within the next 2 to 3 days, there is a relatively rapid onset of mature milk production.24
Breastfeeding
When human milk is unavailable, modern infant formulas are acceptable but incomplete substitutes. Most commonly made from cow’s milk or soybeans, formulas are similar to human milk in terms of water, fat, carbohydrates, and calories, but are devoid of antibodies and other bioactive factors. The exceptional benefits of human breast milk compared to formulas are so numerous that the American Academy of Pediatrics policy statement is that “pediatricians and other health care professionals should recommend human milk for all infants in whom breastfeeding is not specifically contraindicated.”26
Breastfeeding and Anovulation
As a result of these variables, the length of time a breastfeeding woman will have amenorrhea is highly variable, and women should not rely on lactation to prevent pregnancy. As many as 10% of breastfeeding women will ovulate within 10 weeks of giving birth.27 Two thirds of women who breastfeed for more than 9 months will resume ovulation and menstruation during that time. A small fraction of breastfeeding women will remain anovulatory and amenorrheic for more than 1 year.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree