Chapter 19 Anovulation and Ovulatory Dysfunction
INTRODUCTION
Anovulation and ovulatory dysfunction are frequent reasons for gynecologic referral. The most common form of ovulatory dysfunction, polycystic ovary syndrome (PCOS), is estimated to occur in up to 6% to 10% of women.1 Ovulatory dysfunction may result in a spectrum of clinical symptoms ranging from amenorrhea to frequent, irregular, and heavy menses. In addition to menstrual irregularities, many women will experience subfertility or other endocrine symptoms, such as hirsutism. Choice of therapy will depend on numerous factors, including the presenting symptom, age, desire for fertility, and associated conditions.
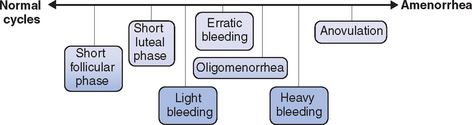
Regardless of the etiology, a spectrum of ovulatory disorders exists that may precede the eventual development of amenorrhea or occur during the recovery from amenorrhea (Fig. 19-1). Underlying abnormalities in ovulatory function may present clinically with either quantitative or qualitative changes in the menstrual cycle. A prolonged follicular phase or intermittent anovulation both result in infrequent (cycles greater than 35 days) and often heavy menstrual flow; a shortened follicular or luteal phase is associated with frequent menstrual flow and often premenstrual spotting.
PATHOPHYSIOLOGY
The Hypothalamus
The pathway ultimately responsible for ovulation is initiated by pulsatile release of gonadotropin-releasing hormone (GnRH) from neurons in the arcuate nucleus of the hypothalamus into the portal circulation. From there, GnRH induces release of the anterior pituitary hormones, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The amplitude and frequency of GnRH release are critical to normal functioning of the HPO axis.2 Numerous hormones and neurotransmitters are known to modulate GnRH release, including dopamine, norepinephrine, neuropeptide Y, and endorphins. Factors affecting release of these hormones and neurotransmitters or the physical delivery of GnRH to the anterior pituitary may ultimately disrupt ovulation.
Excessive cortisol secretion (due to high corticotropin-releasing hormone activity), high levels of endogenous neuropeptide Y and opioids (specifically endorphins) are all thought to play a role in suppression of GnRH activity associated with psychological stress and eating disorders. Less commonly, injury, infection, or central nervous system (CNS) tumors may interfere with delivery of GnRH to the anterior pituitary via the portal circulation. Hypothalamic dysfunction or failure has been reported following severe deceleration injuries that cause pituitary stalk transection, CNS tumors such as craniopharyngiomas or hamartomas that cause stalk compression, and infiltrative diseases such as tuberculosis or sarcoidosis.
More recently, the role of leptin in maintenance of BMI, regulation of appetite, and control of reproductive function has been investigated. Leptin is a 146-amino acid protein hormone expressed in numerous sites but mainly in adipose tissue. It has been shown to have a direct stimulatory effect on GnRH pulsatility, as well as LH/FSH secretion from the anterior pituitary, and is likely central to control of the HPO axis through a number of complex endocrine and paracrine actions.3 Leptin may act as a sensor of energy deficiency, limiting the high-energy cost of reproduction and growth during periods of starvation. Concentrations decrease rapidly with fasting, resulting in suppression of reproductive, thyroid, and growth hormones. A preliminary study suggests that administration of recombinant human leptin may reverse the neuroendocrine abnormalities associated with hypothalamic amenorrhea, inducing a return of hormone secretion reminiscent of puberty.4
Pituitary Gonadotropins
The secretion of LH and FSH in response to GnRH stimulation varies as puberty progresses. As the HPO axis matures, it is common to see abnormalities in ovulatory function. The average time from onset of menses to establishment of regular ovulatory cycles is 2 to 3 years.5
The Ovary
Depletion of follicles is a common cause of abnormalities in ovulatory function during the perimenopausal transition. Early follicular (day 3) FSH levels rise due to the relative resistance of the remaining follicles to FSH-dependent stimulation and a concomitant decrease in serum inhibin. Accelerated follicular development may occur in response to increased FSH levels, with elevated estradiol secretion early in the follicular phase, ovulation as early as day 8 to 11, and corresponding shortening of the menstrual interval (e.g., from a 28-day to a 23-day pattern).6,7 As follicular number and responsiveness decline, the pattern progresses to oligo-ovulation or anovulation and eventual amenorrhea. This inevitable process of reproductive aging usually begins in the late 30s and may become clinically manifest with changing menstrual patterns throughout the 40s.7
A similar process, albeit occurring at an earlier age, may occur in young women who develop premature ovarian failure with loss of ovarian function before age 40.8,9 Some young women with premature ovarian failure experience premature follicular depletion; others likely have a normal complement of follicles that are unresponsive to gonadotropin stimulation, possibly on an autoimmune basis. Young women with premature ovarian failure may also experience sporadic and transient return of ovarian function after diagnosis, ranging from intermittent estrogen-withdrawal bleeding to normal ovulatory cycles and the possibility of conception.10–12
Lastly, the most common cause of ovulatory dysfunction is PCOS, a complex condition of uncertain etiology. PCOS is the most common endocrine disorder in women and is characterized by hyperestrogenic hyperandrogenic chronic anovulation, which may present clinically with various ovulatory and menstrual disorders, infertility, acne, hirsutism, and obesity.13 Numerous metabolic aberrations may also be associated with PCOS, including obesity, insulin resistance, type 2 diabetes, dyslipidemia, and cardiovascular disease. Insulin resistance appears to play a central role in the reproductive dysfunction and long-term health consequences of PCOS.12
When evaluating women with ovulatory dysfunction, it is important to recognize that PCOS is a syndrome with a broad spectrum of clinical phenotypes, and that no existing classification will encompass all possible phenotypes. The Rotterdam PCOS consensus workshop recently published revised criteria for the diagnosis of PCOS.14 After exclusion of other etiologies (congenital adrenal hyperplasia, androgen-secreting tumors, Cushing’s syndrome), two of three of the following criteria must be present to establish a diagnosis of PCOS:
Although frequently associated with PCOS, obesity in itself may contribute to ovulatory dysfunction.15 Numerous studies, including the Nurses’ Health Study, have reported increased rates of ovulatory infertility with increasing BMI.16,17 It is believed that obesity leads to a state of functional hyperandrogenism. Central (abdominal) obesity is associated with insulin resistance and high circulating insulin levels. Because the production of sex hormone-binding globulin (SHBG) in the liver is directly inhibited by insulin, it follows that women with central obesity have lower levels of SHBG and higher levels of free testosterone than age- and weight-matched controls with peripheral obesity.18 This functional hyperandrogenism, in combination with increased peripheral aromatization of estrogen in adipose tissue, leads to alteration in the balance of sex steroids and ultimately contributes to ovulatory dysfunction.
Other Endocrine Disorders
A number of other endocrine disorders may result in ovulatory dysfunction through disruption of the HPO axis. Hyperprolactinemia, for example, leads to suppression of GnRH secretion.19 Similarly, increased thyrotropin-releasing hormone (TRH) activity in women with hypothyroidism ultimately results in reduced GnRH pulsatility through the direct stimulatory effect of TRH on prolactin secretion. Hyperthyroidism may result in a range of menstrual disturbances, from frequent heavy bleeding to amenorrhea.
Congenital Adrenal Hyperplasia
Women with both classic and late onset congenital adrenal hyperplasia (CAH) may present with ovulatory disturbances similar to PCOS.20 Menstrual abnormalities in these women were initially attributed to inadequate glucocorticoid suppression of adrenal androgen production, resulting in suppression of the HPO axis. Particularly in classic CAH, there is evidence of ovarian hyperandrogenism similar to PCOS despite adequate adrenal suppression with dexamethasone.
It has been postulated that perinatal exposure to hyperandrogenism in women with CAH results in hypersecretion of LH in response to GnRH stimulation at puberty. This allows the development of ovarian hyperandrogenism independent of control of adrenal androgen production.21 A similar abnormality has been reported but is less common in late-onset CAH.
Luteal Phase Defect
The role of luteal phase defect in infertility remains controversial, however. Studies designed to address this issue have demonstrated a significant prevalence of luteal phase defect in the fertile population and are hampered by a lack of consensus regarding diagnostic criteria. Performance of repeated endometrial biopsies to assess histology is often impractical and the interpretation of results limited by a high degree of interobserver variability. Similarly there is no agreement on the appropriate normal values for serum progesterone. Finally, lack of a clear treatment effect with progesterone supplementation has led many to question the importance of luteal phase defect as a cause of infertility.24–26
Luteinized Unruptured Follicle
Lastly, luteinized unruptured follicle syndrome has been reported as a transient or ongoing cause of ovulatory disturbance. The syndrome involves absence of sonographic signs of ovulation or lack of an ovulatory stigma at laparoscopy 48 to 72 hours after the LH surge. The phenomena may occur in conjunction with periovulatory nonsteroidal anti-inflammatory ingestion, leading to inhibition of prostaglandin-induced follicular rupture.27,28 Other cases have been reported in conjunction with suboptimal doses or improperly timed human chorionic gonadotropin injections during ovulation induction cycles.29 Aside from these iatrogenic cases, there is no clear etiology, no documented treatment, and considerable controversy over the diagnostic criteria with their inherent variability and observer biases.29,30 A survey of practice patterns in the United States indicated that only 8% of university-based board-certified reproductive endocrinologists would screen for this disorder.31
ESTABLISHING A DIAGNOSIS
Considerable controversy exists over the criteria used to define luteal phase defects.25,32 Histologic dating of a luteal phase endometrial biopsy specimen has been considered the gold standard, requiring a 2- to 3-day difference between histologic and chronologic dating in two separate cycles.22 However, the test is invasive and uncomfortable, and suffers from substantial intraobserver and interobserver variability.33 Serum progesterone measurements are less invasive, although less accepted due to debate over the threshold level used for diagnosis, the number of suboptimal tests needed, and the relatively poor correlation with endometrial response.24,25,34 Progesterone secretion is both pulsatile (in response to episodic LH release) and parabolic (with peak levels in the midluteal phase), so low levels may occur physiologically in the trough prior to each LH pulse and at the beginning and end of each luteal phase.35 The threshold progesterone value used to differentiate ovulatory from anovulatory cycles (6 to 18 nmol/L or 2 to 5 ng/mL) should not be confused with that suggested for the diagnosis of defective luteal phase (21 to 32 nmol/L or 7 to 10 ng/mL).24,34
DIAGNOSIS
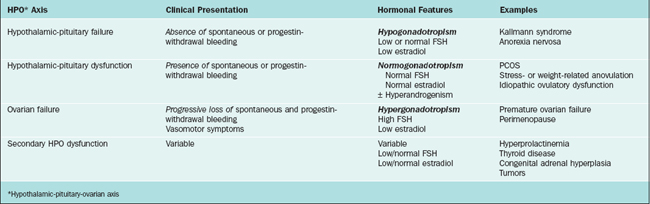
Various classification systems have been developed for ovulatory disorders (Table 19-1). Such systems also provide the basis for a timely and cost-effective evaluation. They revolve around three key principles that can also be used to guide the history, physical examination, investigations, and management (Table 19-2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree